Team:Northwestern/Project
From 2010.igem.org
(Difference between revisions)
| Line 193: | Line 193: | ||
|- | |- | ||
|colspan="2"| | |colspan="2"| | ||
| + | |||
| + | |||
| + | ===Strategy=== | ||
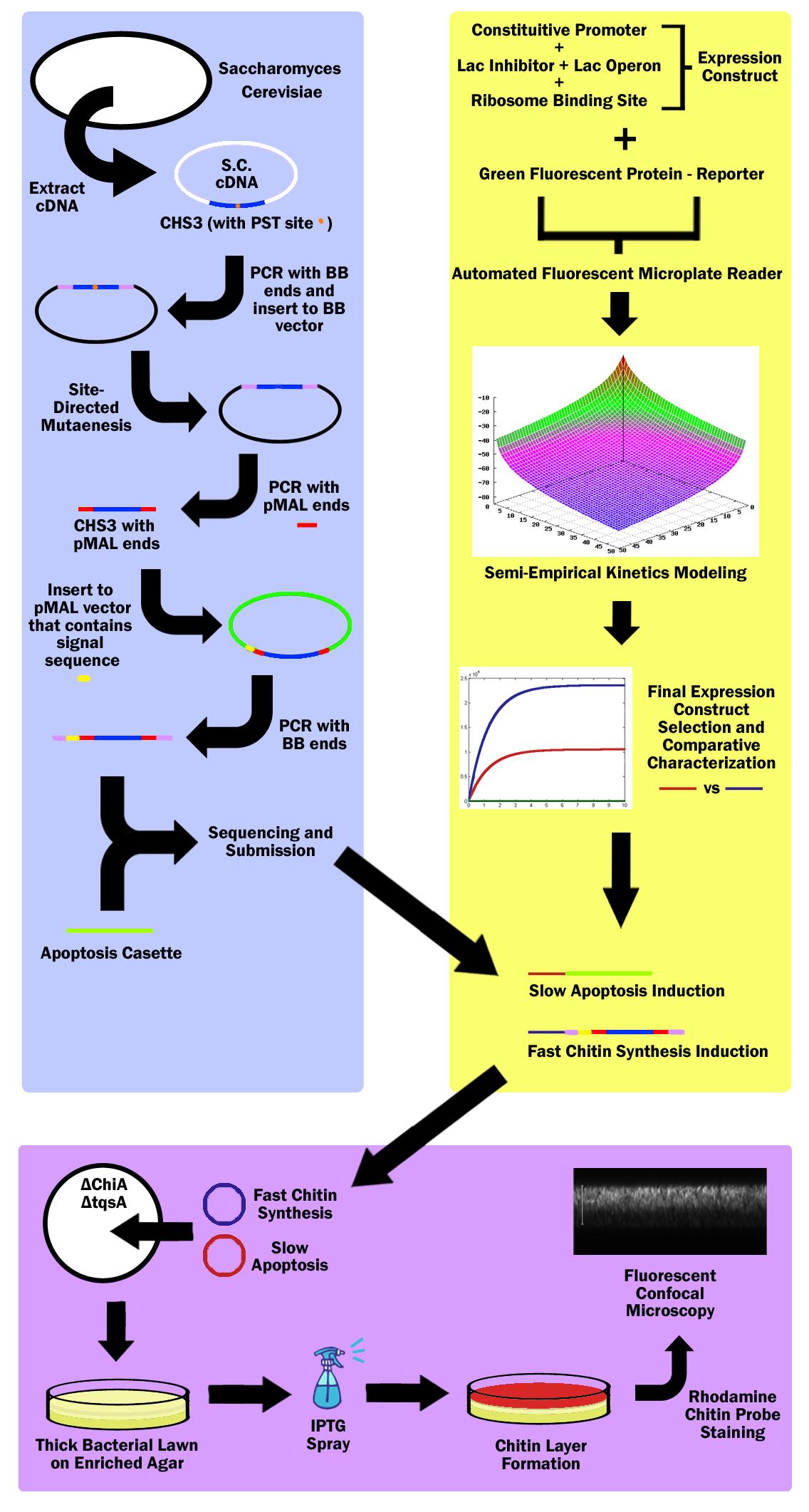
First, the Chitin Synthase 3 (CHS3) gene was subcloned out of Saccharomyces Cerevisiae (Baker’s Yeast) cDNA (complementary DNA – contains no introns, thus can be used in prokaryotes) with biobrick restriction sites (EcoR1, Xbal1 on one end, and Spe1, Pst1 on the other). CHS3 was chosen as it was found to be the major enzyme (knockouts had 80% reduced Chitin) in its family and requires no co-enzyme or activating compounds. Upon running CHS3 through NEB Cutter V2.0, we found a PST1 restriction site which is incompatible with the biobrick standard (EcoR1, Xbal, Spe1, Pst1). To remove this Pst1 site, we inserted CHS3 into a biobrick vector plasmid, and performed site-directed mutagenesis (SDM). | First, the Chitin Synthase 3 (CHS3) gene was subcloned out of Saccharomyces Cerevisiae (Baker’s Yeast) cDNA (complementary DNA – contains no introns, thus can be used in prokaryotes) with biobrick restriction sites (EcoR1, Xbal1 on one end, and Spe1, Pst1 on the other). CHS3 was chosen as it was found to be the major enzyme (knockouts had 80% reduced Chitin) in its family and requires no co-enzyme or activating compounds. Upon running CHS3 through NEB Cutter V2.0, we found a PST1 restriction site which is incompatible with the biobrick standard (EcoR1, Xbal, Spe1, Pst1). To remove this Pst1 site, we inserted CHS3 into a biobrick vector plasmid, and performed site-directed mutagenesis (SDM). | ||
| + | |||
Research revealed that chitin synthase is a transmembrane enzyme, and in order to simulate similar conditions, we decided to use the pMAL vector by NEB which contains a periplasmic membrane signal sequence. CHS3 was subcloned in to the pMAL-p5x NEB vector by first PCR’ing it out of the biobrick vector plasmid - on which it underwent SDM - with pMAL restriction sites (Nde1 and EcoR1) and ligated into the pMAL-p5x vector. Then, the CHS3 was again amplified with biobrick sites, but this time, with the periplasmic signal sequence native to the pMAL-p5x plasmid, and inserted into a standard biobrick vector. The entire part was then sequenced and submitted to the registry of standard parts. | Research revealed that chitin synthase is a transmembrane enzyme, and in order to simulate similar conditions, we decided to use the pMAL vector by NEB which contains a periplasmic membrane signal sequence. CHS3 was subcloned in to the pMAL-p5x NEB vector by first PCR’ing it out of the biobrick vector plasmid - on which it underwent SDM - with pMAL restriction sites (Nde1 and EcoR1) and ligated into the pMAL-p5x vector. Then, the CHS3 was again amplified with biobrick sites, but this time, with the periplasmic signal sequence native to the pMAL-p5x plasmid, and inserted into a standard biobrick vector. The entire part was then sequenced and submitted to the registry of standard parts. | ||
A bacterial apoptosis cassette made by the Brown iGEM team in ‘08 was taken from the igem 2010 distribution kit and colony amplified. | A bacterial apoptosis cassette made by the Brown iGEM team in ‘08 was taken from the igem 2010 distribution kit and colony amplified. | ||
| + | |||
3 constitutive promoters and 2 lac-inhibitor/lac-operon combination parts were taken from the registry and ligated in all 6 combinations through 3A Assembly. These combinations would express different levels of laci, thus exhibiting variable inductivity. These 6 constructs were then ligated with a reporter protein - green fluorescent protein - and characterized via an automated fluorescent microplate reader; fluorescence was used to determine the construct inductivity and expression level. | 3 constitutive promoters and 2 lac-inhibitor/lac-operon combination parts were taken from the registry and ligated in all 6 combinations through 3A Assembly. These combinations would express different levels of laci, thus exhibiting variable inductivity. These 6 constructs were then ligated with a reporter protein - green fluorescent protein - and characterized via an automated fluorescent microplate reader; fluorescence was used to determine the construct inductivity and expression level. | ||
Then the data was used to fit our own theoretical kinetics model that accounts for IPTG diffusion through biofilm, external IPTG concentration, internal IPTG concentration, lac repressor concentration, repressor bound gene (DNA) concentration, unbound gene (DNA) concentration, mRNA concentration, enzyme concentration, substrate concentration, enzyme-substrate complex concentration, and final product concentration, as well as all the rate constants involved. Using the data acquired from the microplate reader, a final semi-empirical kinetics model was generated. Using this model, two different constructs were chosen; both with similar inductivity, but one with and the other without time delay. | Then the data was used to fit our own theoretical kinetics model that accounts for IPTG diffusion through biofilm, external IPTG concentration, internal IPTG concentration, lac repressor concentration, repressor bound gene (DNA) concentration, unbound gene (DNA) concentration, mRNA concentration, enzyme concentration, substrate concentration, enzyme-substrate complex concentration, and final product concentration, as well as all the rate constants involved. Using the data acquired from the microplate reader, a final semi-empirical kinetics model was generated. Using this model, two different constructs were chosen; both with similar inductivity, but one with and the other without time delay. | ||
| + | |||
The fast expression construct was ligated with Chitin Synthase, while the slow expression construct was ligated with the apoptosis cassette. Both plasmids were then transformed into a ChiA and tqsA double knockout strain of K12 Escherichia Coli. ChiA knockouts lack Chitinase, which is an enzyme that catabolizes chitin. tqsA knockouts have a disrupted quorum sensing mechanism that allows them to grow very thick lawns. The mutants were acquired from Keio collection (one from Northwestern, and the other from Yale respectively), and the Multiplex Automated Genome Engineering (MAGE) protocol developed by H. H. Wang. et al. was used to acquire double knockouts. The MAGE protocol is an accelerated evolution procedure where oligonucleotides are electroporated into the cells in order to introduce mutations in the genome during replication by binding as lagging strand primers. | The fast expression construct was ligated with Chitin Synthase, while the slow expression construct was ligated with the apoptosis cassette. Both plasmids were then transformed into a ChiA and tqsA double knockout strain of K12 Escherichia Coli. ChiA knockouts lack Chitinase, which is an enzyme that catabolizes chitin. tqsA knockouts have a disrupted quorum sensing mechanism that allows them to grow very thick lawns. The mutants were acquired from Keio collection (one from Northwestern, and the other from Yale respectively), and the Multiplex Automated Genome Engineering (MAGE) protocol developed by H. H. Wang. et al. was used to acquire double knockouts. The MAGE protocol is an accelerated evolution procedure where oligonucleotides are electroporated into the cells in order to introduce mutations in the genome during replication by binding as lagging strand primers. | ||
| + | |||
The double knockout colonies with both constructs were then sprayed with IPTG, which first induced the fast chitin synthesis construct, which caused a buildup of chitin in cells in the top layer of the bacterial lawn. Then the slow apoptosis construct was induced, which then lysed the cells, and released a layer of chitin in the top layer of the bacterial lawn. This construct was then stained with Chitin-specific rhodamine probe by NEB as well as baclight by Invitrogen in order to ensure that the cells were producing chitin and lysing. The lawn was then imaged through a fluorescent confocal microscope. | The double knockout colonies with both constructs were then sprayed with IPTG, which first induced the fast chitin synthesis construct, which caused a buildup of chitin in cells in the top layer of the bacterial lawn. Then the slow apoptosis construct was induced, which then lysed the cells, and released a layer of chitin in the top layer of the bacterial lawn. This construct was then stained with Chitin-specific rhodamine probe by NEB as well as baclight by Invitrogen in order to ensure that the cells were producing chitin and lysing. The lawn was then imaged through a fluorescent confocal microscope. | ||
|} | |} | ||
Revision as of 02:01, 23 October 2010
| Home | Brainstorm | Team | Acknowledgements | Project | Human Practices | Parts | Notebook | Calendar | Protocol | Safety | Links | References | Media | Contact |
|---|
 "
"