Team:Washington/Gram Negative
From 2010.igem.org
(→The Type VI Secretion System) |
(→The Type VI Secretion System) |
||
| Line 31: | Line 31: | ||
=The Type VI Secretion System= | =The Type VI Secretion System= | ||
| - | [[Image:UWT6SSSchematic.png|300px|right|thumb|T6SS Creates a Channel that Secretes Proteins from Host Cell into Target Cell]] The Type VI Secretion System (T6SS) is an injection mechanism found in many Gram-negative bacteria (such as ''Pseudomonas aeruginosa''). The T6SS acts much like a spear by physically puncturing the cell membrane. After puncturing, the "spear" provides a channel through which proteins, such as toxins, can be inserted into the punctured cell. We wanted to put this system into a model bacterium and use it to target Gram-negative pathogens. The T6SS is physically incapable of puncturing the cell membrane of gram-positive bacteria, or the cell membrane of eukaryotic cells. This makes the T6SS a perfect candidate for a probiotic because it is unable to harm either human cells or helpful gram-positive bacteria. This system has been characterized in the lab of [http://depts.washington.edu/micro/faculty/mougous.htm Dr. Joseph Mougous]. ''E. coli'' is one bacterial species that does not contain the T6SS. Since ''E. coli'' is amenable to genetic changes, is easy to work with in the lab, and is a common gram-negative bacteria found naturally in the gut, it seemed only logical to move the T6SS into it. | + | [[Image:UWT6SSSchematic.png|300px|right|thumb|'''T6SS Creates a Channel that Secretes Proteins from Host Cell into Target Cell''']] The Type VI Secretion System (T6SS) is an injection mechanism found in many Gram-negative bacteria (such as ''Pseudomonas aeruginosa''). The T6SS acts much like a spear by physically puncturing the cell membrane. After puncturing, the "spear" provides a channel through which proteins, such as toxins, can be inserted into the punctured cell. We wanted to put this system into a model bacterium and use it to target Gram-negative pathogens. The T6SS is physically incapable of puncturing the cell membrane of gram-positive bacteria, or the cell membrane of eukaryotic cells. This makes the T6SS a perfect candidate for a probiotic because it is unable to harm either human cells or helpful gram-positive bacteria. This system has been characterized in the lab of [http://depts.washington.edu/micro/faculty/mougous.htm Dr. Joseph Mougous]. ''E. coli'' is one bacterial species that does not contain the T6SS. Since ''E. coli'' is amenable to genetic changes, is easy to work with in the lab, and is a common gram-negative bacteria found naturally in the gut, it seemed only logical to move the T6SS into it. |
<br> | <br> | ||
Revision as of 01:07, 21 October 2010
New and Effective Killer of Gram-Negative Bacteria
It is becoming increasingly apparent that the utilization of small molecule antibiotics is becoming out-dated and less effective as time passes. Pathogens are continually evolving resistance to currently used antibiotics, and the discovery or modification of antibiotic treatments are slow to match this resistance, resulting in antibiotics that are less effective if not completely futile. Another problem with today's antibiotics is that they do not differentiate between pathogenic and non-pathogenic bacteria - they kill both. There are many common diseases caused by Gram-negative bacteria which inhabit the human gut, such as, but not limited to: cholera (Vibrio cholerae), dysentery (Shigella), and food poisoning (Salmonella Typhimurium). When these diseases are often treated with antibiotics, but in addition to killing pathogens (unless resistance has been obtained), these antibiotics also kill other bacteria naturally found in the gut, causing a diverse range of side effects. Many of the bacteria in the gut are advantageous: they aid the body in digestion, production of vitamins such as vitamin K, and competitively exclude pathogenic invaders. Both problems of resistance and non-specificity could be lessened by an antibacterial agent that selectively kills pathogenic bacteria. This would limit the chance of the development of resistance by limiting exposure, and would drastically decrease damage to the helpful gut flora. The goal of this project is to turn the natural bacterial weapon to kill other bacteria, the Type VI secretion system / toxin injection system, into a probiotic that specifically targets a pathogenic gram-negative bacteria and is activated only when that specific bacteria is present.
The Type VI Secretion System
The Type VI Secretion System (T6SS) is an injection mechanism found in many Gram-negative bacteria (such as Pseudomonas aeruginosa). The T6SS acts much like a spear by physically puncturing the cell membrane. After puncturing, the "spear" provides a channel through which proteins, such as toxins, can be inserted into the punctured cell. We wanted to put this system into a model bacterium and use it to target Gram-negative pathogens. The T6SS is physically incapable of puncturing the cell membrane of gram-positive bacteria, or the cell membrane of eukaryotic cells. This makes the T6SS a perfect candidate for a probiotic because it is unable to harm either human cells or helpful gram-positive bacteria. This system has been characterized in the lab of [http://depts.washington.edu/micro/faculty/mougous.htm Dr. Joseph Mougous]. E. coli is one bacterial species that does not contain the T6SS. Since E. coli is amenable to genetic changes, is easy to work with in the lab, and is a common gram-negative bacteria found naturally in the gut, it seemed only logical to move the T6SS into it.
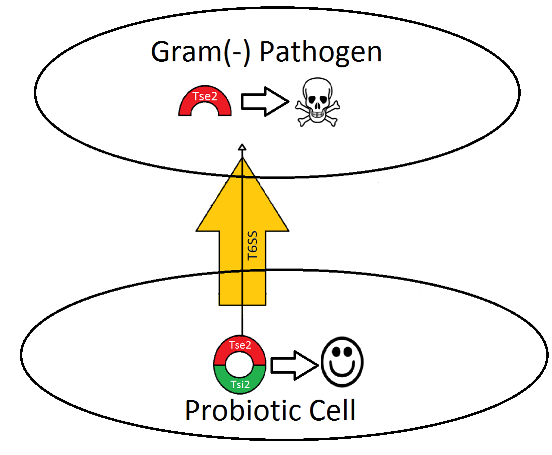
Tse2/ Tsi2 toxin/antitoxin system
In Pseudomonas aeruginosa, one of the major proteins secreted by the T6SS into target Gram-negative bacteria is the toxic protein Tse2. Normally, Tse2(red in figure 2) forms a complex with Tsi2 (green in figure 2), which is a protein coexpressed with Tse2 that serves as an antitoxin. Cells that express Tse2 and Tsi2 are immune to the toxic effects of Tse2. Before Tse2 is secreted into the target cell Tsi2 and Tse2 become unbound and Tse2 is secreted into the target cell by the T6SS. The presence of Tse2 in the absence of Tsi2 causes the target cell to die. By controlling the expression of Tse2 and Tsi2 production and having it turn on only when a pathogen is present, we have an antibiotic that is fairly selective only for the pathogen. This helps combat both problems of specificity and resistance.
 "
"