Team:SDU-Denmark/labnotes9
From 2010.igem.org
(Difference between revisions)
(→Insertion of PS in pSB1C3 and pSB1AK3) |
(→Colony PCR of FlhDCmut in pSB1AK3 and pSB1C3) |
||
| (15 intermediate revisions not shown) | |||
| Line 8: | Line 8: | ||
== Flagella Group == | == Flagella Group == | ||
| + | |||
| + | === Two-step PCR of miniprep === | ||
| + | <br> | ||
| + | ==== First step: PCR of miniprep with mutation primers ==== | ||
| + | <br> | ||
| + | ''Done by:'' Louise <br> | ||
| + | ''Date:'' September 7th <br> | ||
| + | ''Protocol:'' [[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP1.1]] <br> | ||
| + | ''Notes:'' <br> | ||
| + | '''3 x Premix 1:''' <br> | ||
| + | 114.3ul water <br> | ||
| + | 15ul Pfu Buffer <br> | ||
| + | 4.5ul dNTP <br> | ||
| + | 3ul MgSO4 <br> | ||
| + | 4.5ul FlhDC fw <br> | ||
| + | 4.5ul FlhDCmut rev <br> | ||
| + | 1.2ul PFU <br> | ||
| + | 3ul template (miniprep) <br><br> | ||
| + | '''3 x Premix 2:''' <br> | ||
| + | 114.3ul water <br> | ||
| + | 15ul Pfu Buffer <br> | ||
| + | 4.5ul dNTP <br> | ||
| + | 3ul MgSO4 <br> | ||
| + | 4.5ul FlhDCmut fw <br> | ||
| + | 4.5ul FlhDC rev <br> | ||
| + | 1.2ul PFU <br> | ||
| + | 3ul template (miniprep) <br><br> | ||
| + | ''Results:'' | ||
| + | <br> | ||
| + | NanoDrop: <br> | ||
| + | '''Sample 1.1:''' Concentration: 359.5ng/ul Purity: 1.83/2.20 <br> | ||
| + | '''Sample 2.1:''' Concentration: 304.1ng/ul Purity: 1.85/2.23 <br> | ||
| + | <br> | ||
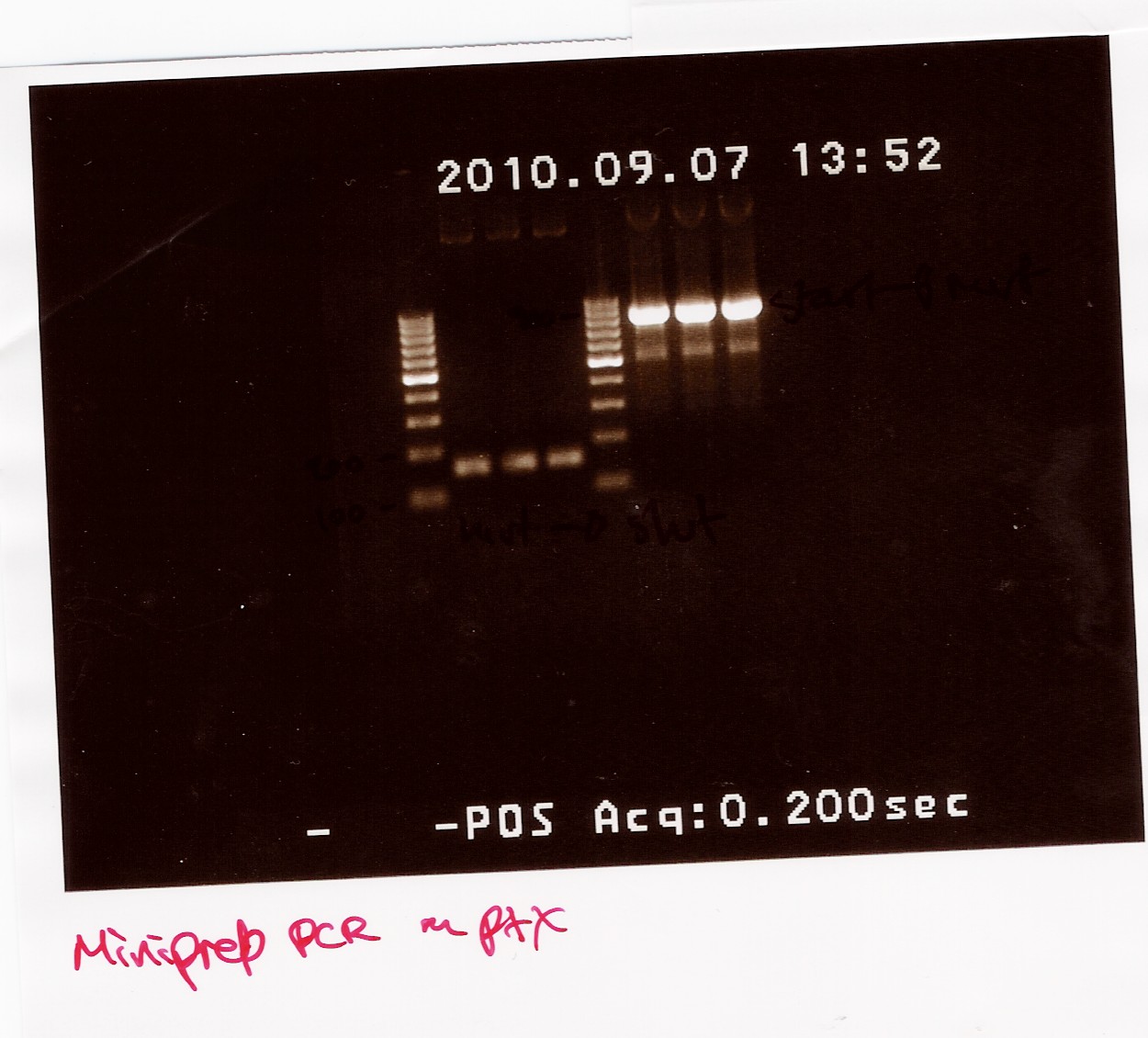
| + | The PCR samples were run on a 1.5% gel with a 100bp-1000bp ladder. The first three samples are all positioned between 100bp and 200bp, this is the part of the gene run with FlhDCmut fv and FlhDC rev primers. The other three samples are all positioned betveen 900bp and 1000bp, this is the pcr with FlhDC fv and FlhDCmut rev primers. All samples looked okay and were pooled as sample 1.1 (light band) and sample 2.1 (heavy band)<br> | ||
| + | [[Image:Team SDU-Denmark-Pfx pcr af miniprep.jpg|300px]] | ||
| + | <br> | ||
| + | A gel extraction of the PCR product of sample 1.1 and 2.1 were made: | ||
| + | <br> | ||
| + | [[Image:Team SDU-Denmark Gel extraction af FlhDCmut dele.jpg|300px]] | ||
| + | <br> | ||
| + | The gel extraction products were used in the preceding PCRs. | ||
| + | |||
| + | ==== Second step: 2-step PCR with mutated template sample 1.1 and 2.1 and FlhDC fw and rev primers ==== | ||
| + | <br> | ||
| + | ''Done by:'' Maria <br> | ||
| + | ''Date:'' September 9th <br> | ||
| + | ''Protocol:'' [[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP1.1]] <br> | ||
| + | ''Notes:'' <br> | ||
| + | '''Premix:''' <br> | ||
| + | 38ul water <br> | ||
| + | 5ul PFU buffer + MgSO4 <br> | ||
| + | 1.5ul dNTP <br> | ||
| + | 1ul Sample 1.1 <br> | ||
| + | 1ul Sample 2.1 <br> | ||
| + | 1.5ul FlhDC fw primer <br> | ||
| + | 1.5ul FlhDC rev primer <br> | ||
| + | 0.5ul PFU <br><br> | ||
| + | '''PCR Program:''' <br> | ||
| + | <table style="text-align: left; height: 260px; width: 225px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">1:Start<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">95C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">2 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">2: Denaturing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">95C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">30 sec<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">3: Annealing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">56C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">30 sec<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">4: Elongation<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">72C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">2 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">5:<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">GO TO<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">2 rep. 4x<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">6: Denaturing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">95C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">30 sec<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">7: Annealing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">63C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">30 sec<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">8: Elongation<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">72C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">2 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">9:<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">GO TO<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">6 rep. 25x<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">10: End <br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">72C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">5 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">12: Hold<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">4C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;"><br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> <br><br> | ||
| + | |||
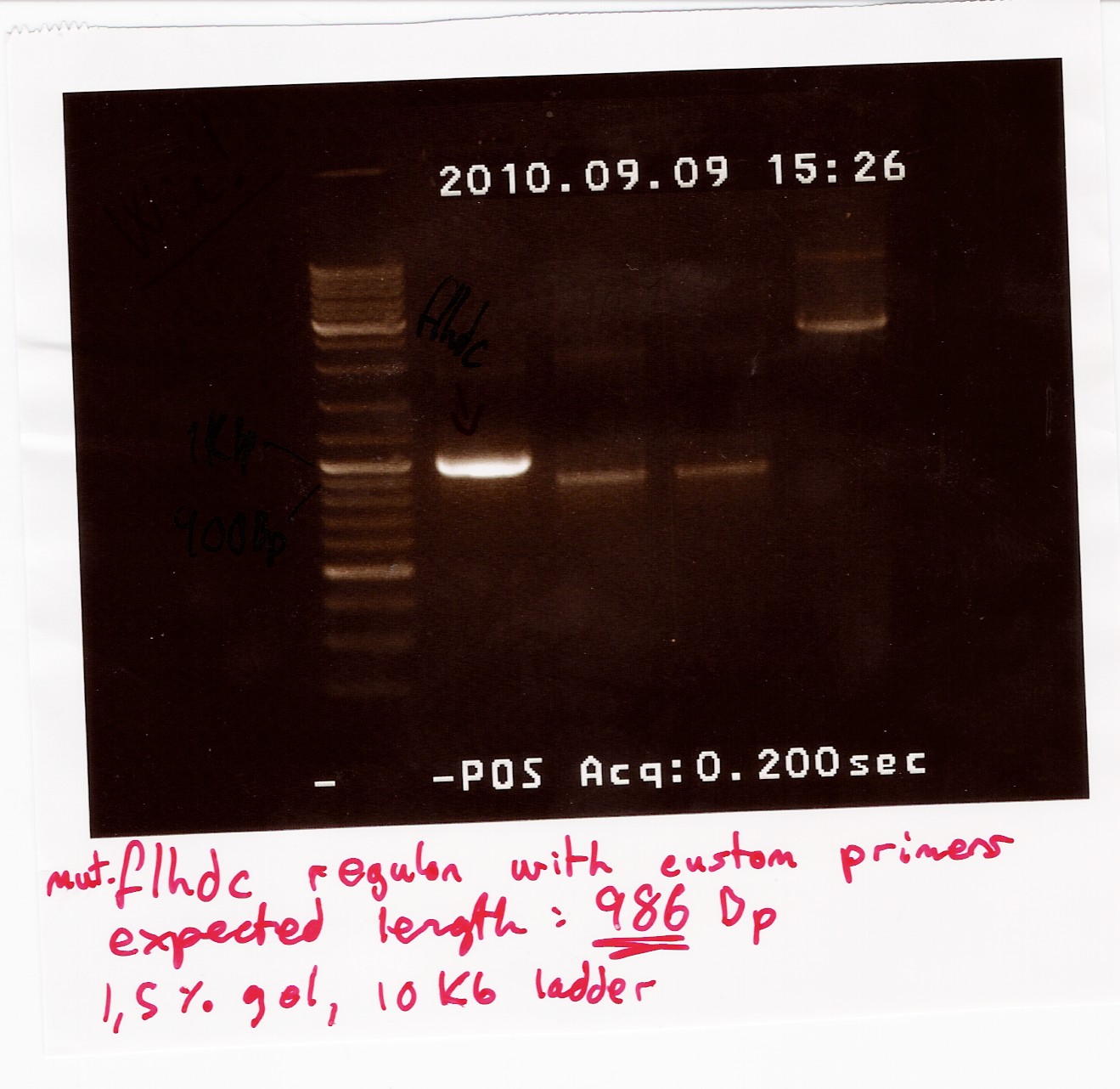
| + | ''Results:'' <br> | ||
| + | Some of the PCR product was run on a 1.5% gel with a 10kb ladder. <br> | ||
| + | [[Image:Team SDU-Denmark God pcr af FlhDCmut (maria) 1.jpg|300px]] | ||
| + | <br> | ||
| + | The rest of the product was extracted from a new gel, unfortunately there were problems with the camera, so there is no picture of the gel extraction. | ||
| + | <br> | ||
| + | ===== PCR of Gel extraction ===== | ||
| + | <br> | ||
| + | ''Done by:'' Maria <br> | ||
| + | ''Date:'' September 9th <br> | ||
| + | ''Protocol:'' [[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP1.1]] <br> | ||
| + | ''Notes:'' <br> | ||
| + | '''Premix x 6:''' <br> | ||
| + | 234ul water <br> | ||
| + | 30ul PFU buffer + MgSO4 <br> | ||
| + | 9ul dNTP <br> | ||
| + | 9ul FlhDC fw primer <br> | ||
| + | 9ul FlhDC rev primer <br> | ||
| + | 2.5ul PFU <br> | ||
| + | 6ul template <br> | ||
| + | |||
| + | <table style="text-align: left; height: 260px; width: 225px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">1:Start<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">95C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">2 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">2: Denaturing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">95C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">30sec<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">3: Annealing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">63C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">30 sec<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">4: Elongation<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">72C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">2 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">5:<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">GO TO<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">2 rep. 29x<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">6: End <br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">72C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">5 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">7: Hold<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">4C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;"><br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> <br><br> | ||
| + | <br> | ||
| + | <br> | ||
| + | ''Results:'' | ||
| + | <br> | ||
| + | The PCR worked fine, unfortunately there were problems with the camera, so there is no picture of the gel. <br> | ||
| + | |||
=== Restriction Digest of Gel Extracted FlhDCmut, pSB1C3 and pSB1AK3 === | === Restriction Digest of Gel Extracted FlhDCmut, pSB1C3 and pSB1AK3 === | ||
| Line 177: | Line 413: | ||
'''Results:''' <Br> | '''Results:''' <Br> | ||
<Br><Br> | <Br><Br> | ||
| + | === Colony PCR of FlhDCmut in pSB1AK3 and pSB1C3 === | ||
| + | <br> | ||
| + | '''Date:''' 9/15 2010<Br> | ||
| + | '''Done By:''' Louise<Br> | ||
| + | '''Protocol:''' [https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.3 CP1.3]<Br> | ||
| + | '''Notes:''' We made 8 C-PCRs with colonies transformed with pSB1AK3 and 7 C-PCRs with colonies transformed with pSB1C3 (we made 8 but due to a mishab sample 5 was not run. Taq polymerase, VF2- and VR primers were used. Only Elongation time was altered from 2 min in the protocol to 1.5 min<Br> | ||
| + | '''Results:''' <Br> | ||
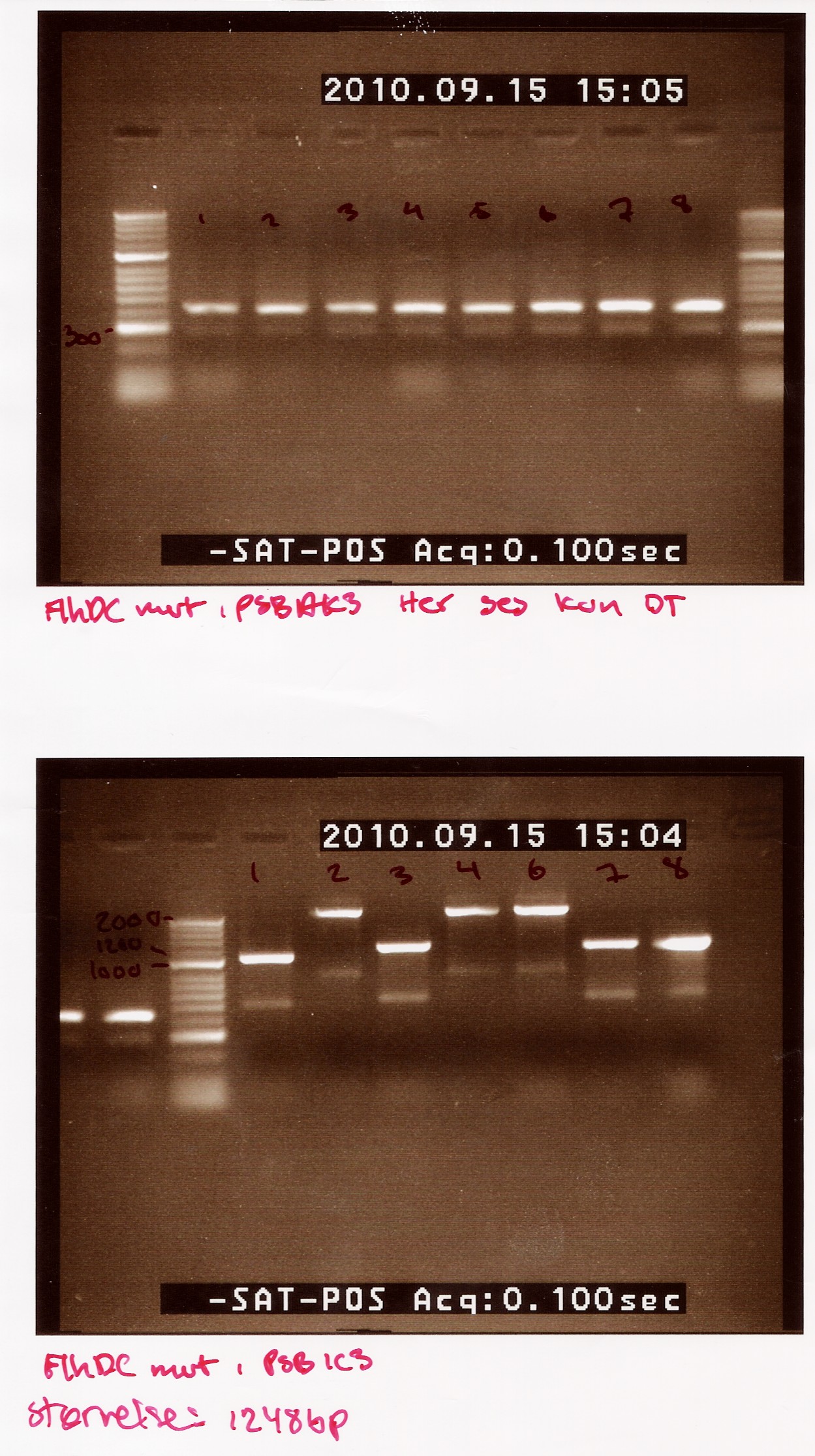
| + | The picture first picture is of FlhDCmut in pSB1AK3. This shows one band at about 400bp, the double terminator. This indicates that non of the colonies had the right plasmid.<br> | ||
| + | The second picture is of FlhDCmut in pSB1C3.This shows the 4 of the 7 colonies had plasmids containing the right incert (sample 1,3, 7 and 8). These samples show a band at approximately 1200bp (FlhDCmut in pSB1C3 is 1248bp). the three other samples show a band heavier than 2000bp <br> | ||
| + | [[Image:Team SDU-Denmark FlhDCmut indsat 1. gang i 1AK3 og 1C3.jpg|300px]] | ||
| + | <br> | ||
| + | ---- | ||
| + | === Colony PCR of FlhDCmut in pSC1AK3 === | ||
| + | <br> | ||
| + | '''Date:''' 9/15 2010<Br> | ||
| + | '''Done By:''' Louise<Br> | ||
| + | '''Protocol:''' [https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.3 CP1.3]<Br> | ||
| + | '''Notes:''' We made 8 C-PCRs with colonies transformed with pSB1AK3 and 7 C-PCRs with colonies transformed with pSB1C3 (we made 8 but due to a mishab sample 5 was not run. Taq polymerase, VF2- and VR primers were used. Only Elongation time was altered from 2 min in the protocol to 1.5 min<Br> | ||
| + | '''Results:''' <Br> | ||
== Photosensor group == | == Photosensor group == | ||
| Line 750: | Line 1,005: | ||
--[[User:Tipi|Tipi]] 13:41, 26 September 2010 (UTC)<br><br> | --[[User:Tipi|Tipi]] 13:41, 26 September 2010 (UTC)<br><br> | ||
| - | ==== Colony PCR of transformation of PS | + | ==== Colony PCR of transformation of PS in pSB1C3 and pSB1AK3 in TOP10 E.Coli ==== |
'''Date:''' 09/11<br> | '''Date:''' 09/11<br> | ||
'''Done by:''' LC & Maria<br> | '''Done by:''' LC & Maria<br> | ||
| Line 829: | Line 1,084: | ||
<br> | <br> | ||
Results: For the ligation into pSB1C3, colonies B, F and H showed bands at the right length. For the other reatcion (in pSB1AK3), colonies A to D showed bands with the correct length. The colonies with bands at the right length will be miniprepped and sent for sequencing.<br> | Results: For the ligation into pSB1C3, colonies B, F and H showed bands at the right length. For the other reatcion (in pSB1AK3), colonies A to D showed bands with the correct length. The colonies with bands at the right length will be miniprepped and sent for sequencing.<br> | ||
| + | [[Image:Team-SDU-Denmark-CPCRPS.jpg|400px]] | ||
| + | <br> | ||
| + | |||
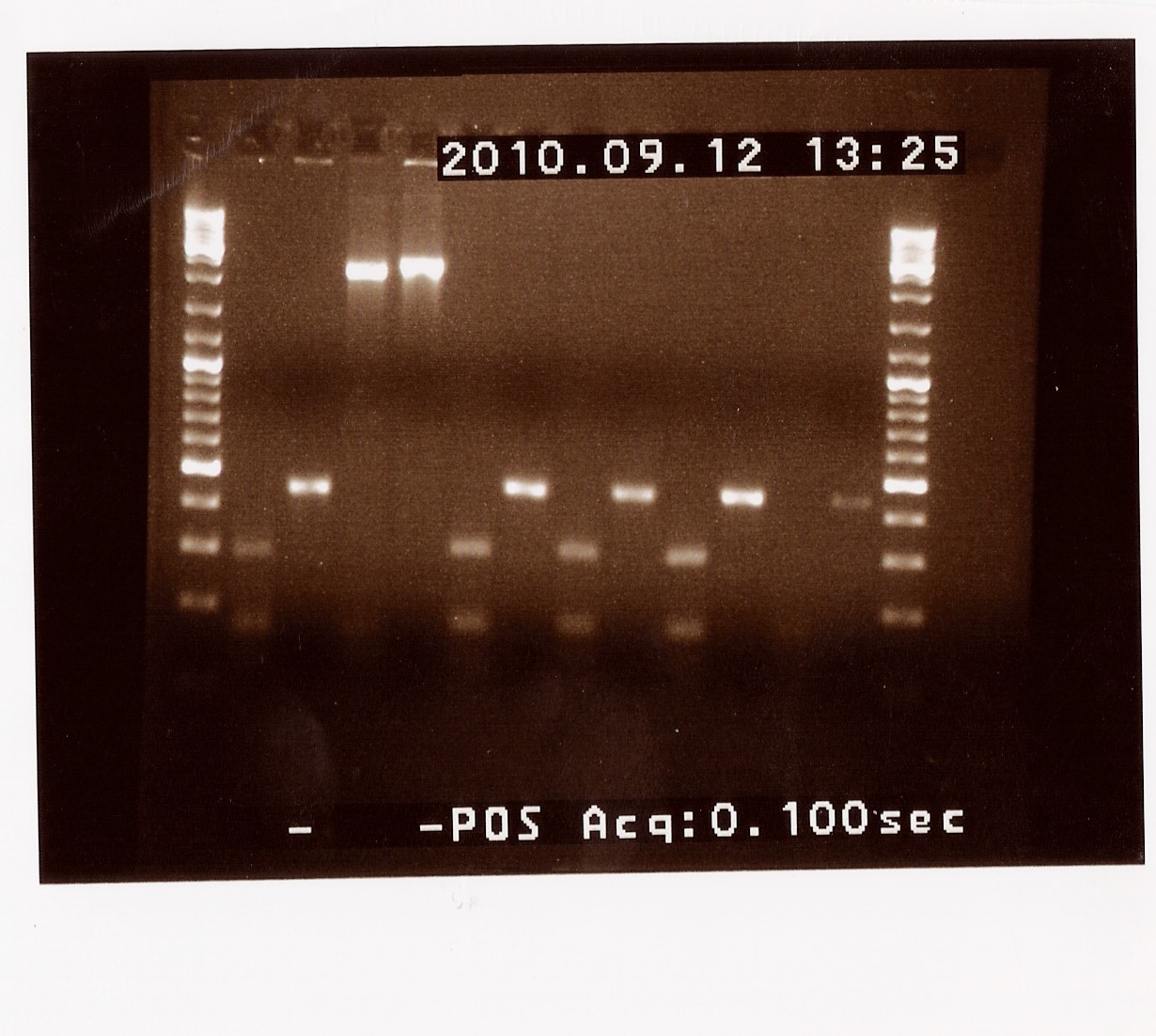
| + | ==== Control restriction digest of cPCR product of PS in pSB1C3 and pSB1AK3 ==== | ||
| + | '''Date:''' 09/12<br> | ||
| + | '''Done by:''' LC & Maria<br> | ||
| + | '''Methods:''' Restriction digest<br> | ||
| + | '''Protocols:''' RD1.1[https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1]<br> | ||
| + | '''Notes:''' <br> | ||
| + | The afore mentioned samples (B, F and H of PS in pSB1C3 and A and D in pSB1AK3) were cut with EcoRI and PstI to see if it was 1) possible to cut them at all, thereby confirming the presence of biobrick prefix and suffix and 2) determining if the insert in the plasmid has the right length. <br> | ||
| + | Mix for 1 RD reaction:<br> | ||
| + | 12 ul H2O<br> | ||
| + | 1 ul PstI<br> | ||
| + | 1 ul EcoRI <bR> | ||
| + | 2 ul Fast digest green buffer<br> | ||
| + | 5 ul PCR product<br> | ||
| + | <br> | ||
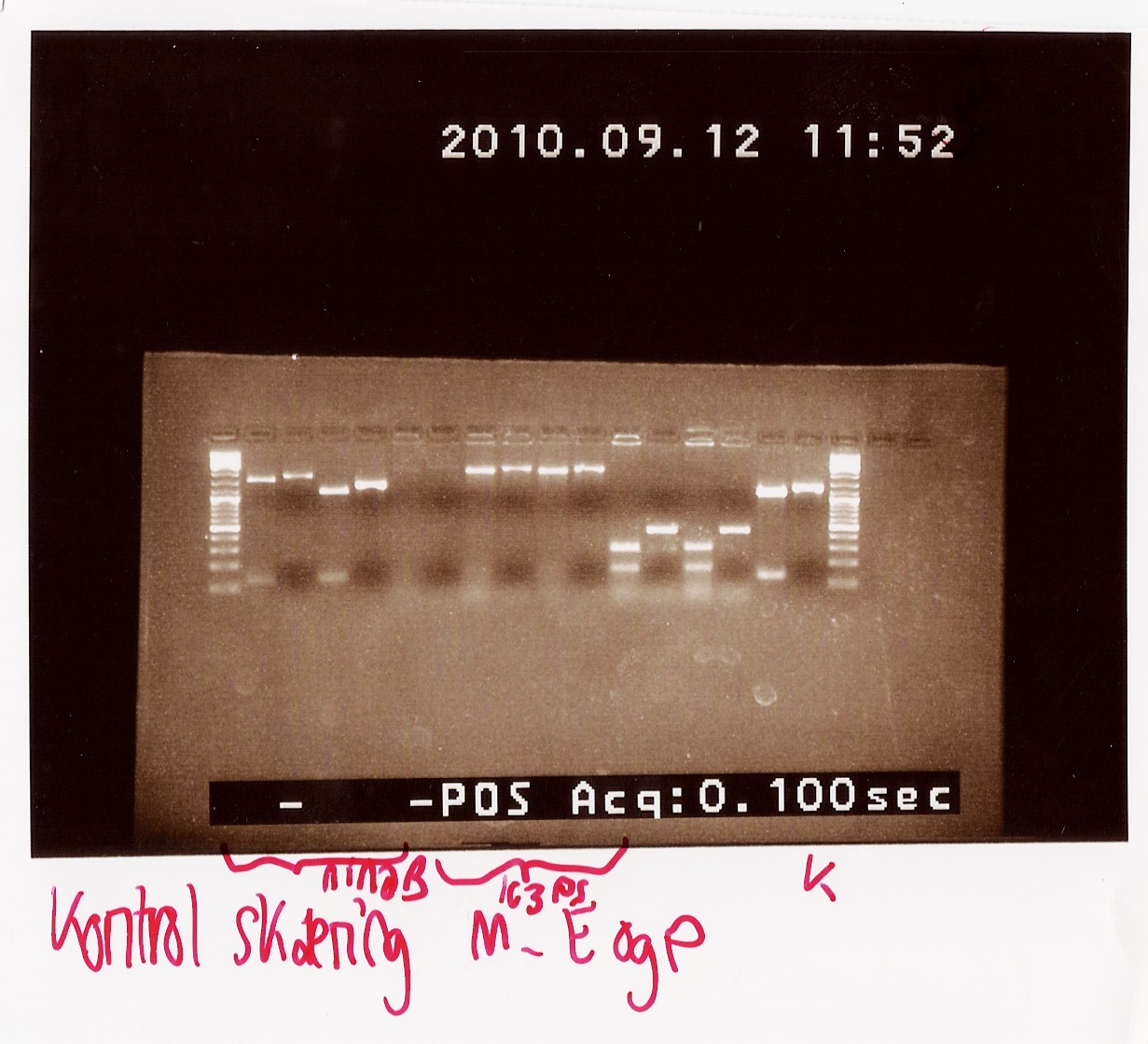
| + | Uncut PCR product and RFP were also loaded on the gel as a control.<br> | ||
| + | <br> | ||
| + | Results: All samples pSB1C3 samples were cut as expected (to around 2000 BP), but the restriction digest of pSB1AK3 sample A and D failed, so that one will have to be repeated with sample B and C, as these only showed a cut double terminator without the PS insert.<br> | ||
| + | [[Image:Team-SDU-Denmark-RDPS.jpg|400px]] | ||
| + | <br> | ||
| + | |||
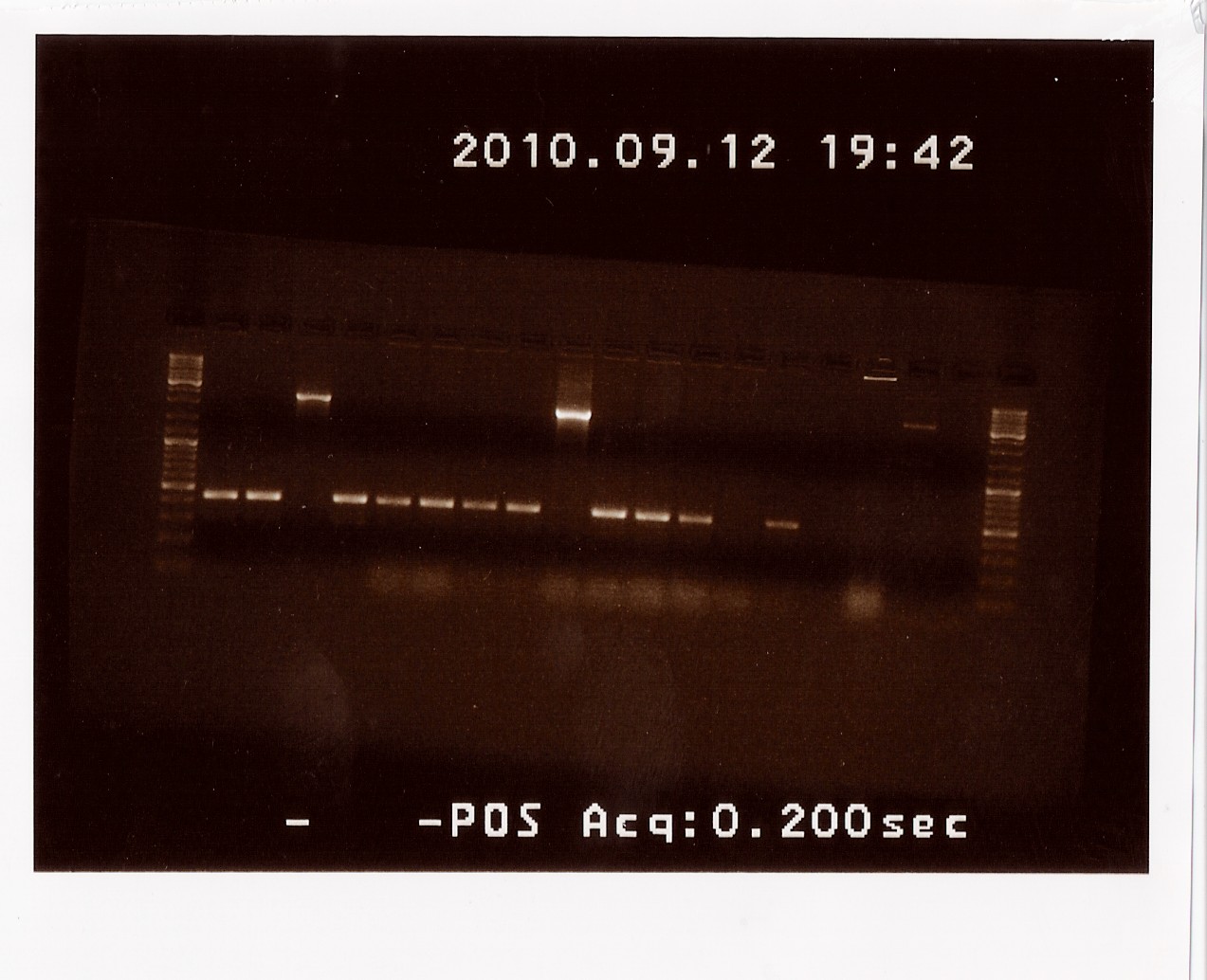
| + | ==== Second control restriction digest of cPCR product of PS pSB1AK3 ==== | ||
| + | '''Date:''' 09/12<br> | ||
| + | '''Done by:''' LC & Maria<br> | ||
| + | '''Methods:''' Restriction digest<br> | ||
| + | '''Protocols:''' RD1.1[https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1]<br> | ||
| + | Since samples A and D fromt he colony PCR gave inadequate results when cut with EcoRI and PstI, we had to repeat the restriction digest with the remaining two samples that were the right length on the cPCR, sample B and C. Samples E, F and G were also cut to confirm that there were no errors in naming the samples that resulted in sample A and D not giving the expected results. <br> | ||
| + | Restriction mixture:<br> | ||
| + | 72 ul H2O<br> | ||
| + | 6 ul PstI<br> | ||
| + | 6 ul EcoRI <bR> | ||
| + | 12 ul Fast digest green buffer<br> | ||
| + | 5 ul PCR product per sample (x6)<br> | ||
| + | <br> | ||
| + | Uncut PCR product was loaded on the gel as a control.<br> | ||
| + | <br> | ||
| + | Results: Only sample C showed bands at the correct length (around 2180 BP), where as all other bands were at the length of a double terminator (b0015) without the photosensor insert. All samples were properly cut indicating the presence of biobrick pre- and suffix in these. Sample C will be miniprepped and sent off to sequencing. <br> | ||
| + | [[Image:Team-SDU-Denmark-RDPS2.jpg|400px]] | ||
| + | <br> | ||
| + | |||
| + | ==== Second round of colony PCR on PS ligated into pSB1AK3 (containing double terminator ==== | ||
| + | '''Date:''' 09/12<br> | ||
| + | '''Done by:''' LC & Maria<br> | ||
| + | '''Methods:''' Colony PCR<br> | ||
| + | '''Protocols:''' CP1.3[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.3]<br> | ||
| + | '''Notes:''' <br> | ||
| + | Since only sample C from the previous colony PCR looked right after a restriction digest we decided to run another round of colony PCR on the photosensor ligated into pSB1AK3, to find more colonys containing the right (ligated!) plamid.<br> | ||
| + | The original samples A - D were also used as a template, now called I, J, K and L. Sample M to Y were new colonies. | ||
| + | <br><br> | ||
| + | Premix:<br> | ||
| + | 42,5 µl 10xTAQ Buffer<br> | ||
| + | 17 µl MgCl2 <br> | ||
| + | 17 µl VF2 <br> | ||
| + | 17 µl VR <br> | ||
| + | 8,5 µl dNTP<br> | ||
| + | 59,5 µl H2O<br> | ||
| + | 2,125 µl TAQ Polymerase<br><br> | ||
| + | The chosen colonies were lysed in 15 ul of H20.<br> | ||
| + | <br> | ||
| + | PCR Program:<br> | ||
| + | <table style="text-align: left;" border="1" cellpadding="2" | ||
| + | cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Start<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">94 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Denaturing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">94 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Annealing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">55 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Elongation<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">72 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2:30 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Goto2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">rep<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">29x<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">End<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">72 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">5 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Hold<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">4 C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;"><br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <br> | ||
| + | <br> | ||
| + | Results: Samples K (former C) and Q produced bands at the right length of around 2150 BP, which eans that sample Q will also be miniprepped and send to sequencing together with the previously chosen sample C (now K).<br> | ||
| + | [[Image:Team-SDU-Denmark-CPCRBST.jpg|400px]] | ||
| + | <br> | ||
=== Miniprep and controle digestion of PS in pSB1C3 and pSB1AK3 === | === Miniprep and controle digestion of PS in pSB1C3 and pSB1AK3 === | ||
| Line 974: | Line 1,355: | ||
In the lanes containing the digested pSB1C3 plasmids bands at app. 2000bp are observed corresponding to PS. The lane containing digested pSB1AK3 plasmids shows a band at app. 2100bp corresponding to the size of PS and the double terminator. Miniprep of coloni B, F and C are sent to sequentation.<br> | In the lanes containing the digested pSB1C3 plasmids bands at app. 2000bp are observed corresponding to PS. The lane containing digested pSB1AK3 plasmids shows a band at app. 2100bp corresponding to the size of PS and the double terminator. Miniprep of coloni B, F and C are sent to sequentation.<br> | ||
--[[User:Tipi|Tipi]] 14:44, 26 September 2010 (UTC)<br><br> | --[[User:Tipi|Tipi]] 14:44, 26 September 2010 (UTC)<br><br> | ||
| + | |||
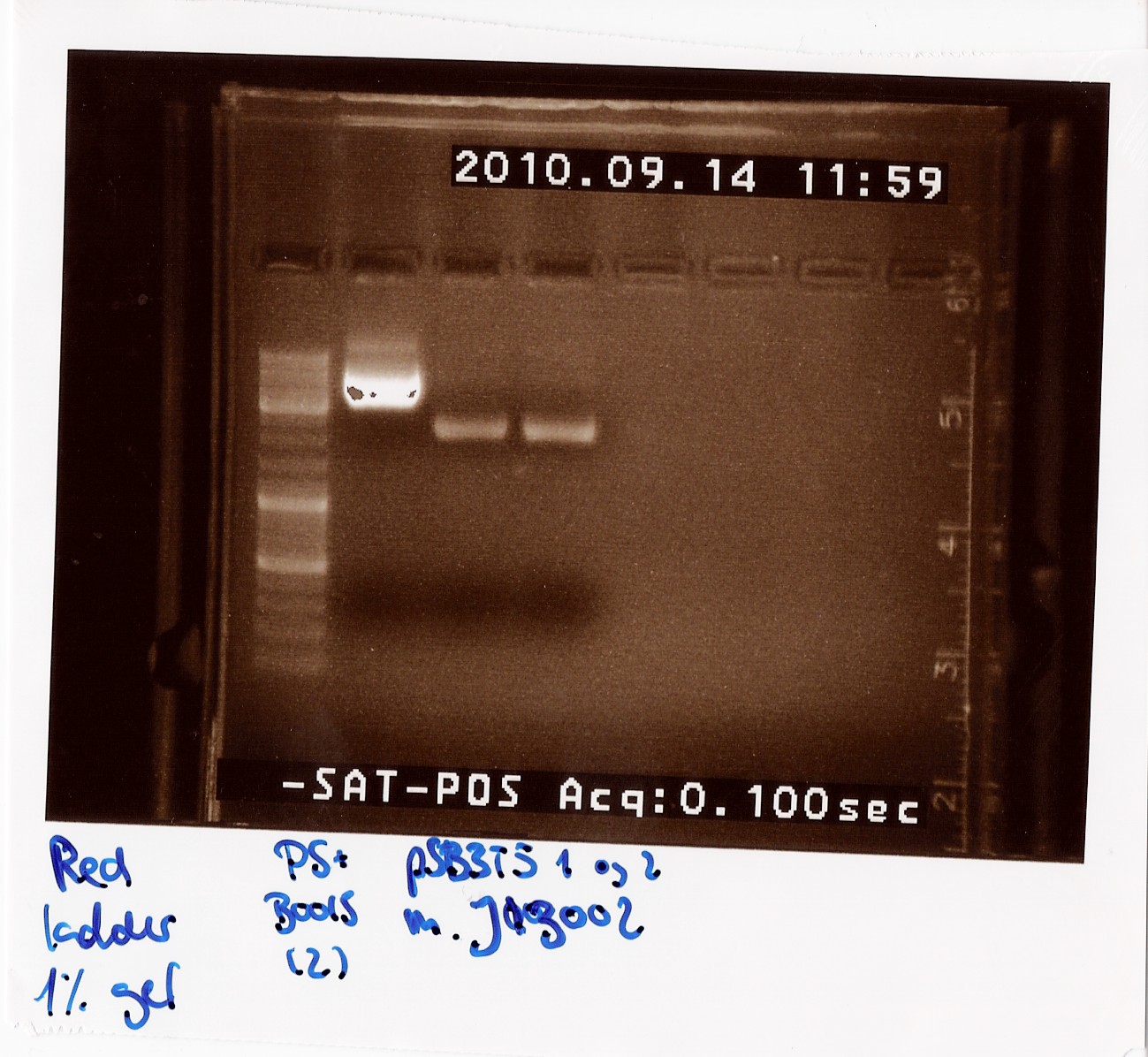
| + | === Miniprep of PS+B0015 sample Q and pSB3T5 containing J13002 === | ||
| + | '''Date: 27/7'''<br> | ||
| + | '''Methods:''' ON, Miniprep<br> | ||
| + | '''Protocol:''' MP1.1<br> | ||
| + | '''Experiment done by:''' LC & Maria<br> | ||
| + | '''Notes:''' PS+B0015 sample Q (1) was eluded in 20 ul to get a concentration over 200 ng/ul, so we would directly be able to send it in for sequencing. All other samples were eluded in 50 ul of elution buffer. <br><br> | ||
| + | '''Results:''' The concentrations were as followed:<br> | ||
| + | PS+B0015 (1): 180 ng/ul<br> | ||
| + | PS+B0015 (2): 130 ng/ul<br> | ||
| + | pSB3T5+J13002 (1 and 2): 50 ng/ul<br> | ||
| + | <br> | ||
| + | Sample Q did not fulfill DNA technologies sample specifications for sequencing we waited with sending it in, since we were expecting the results from sample C which was sent in earlier. Since those returned positive, sample Q was never sent in, since we just proceeded with sample C. | ||
| + | <br> | ||
| + | [[Image:Team-SDU-Denmark-MINIPST.jpg|400px]]<br> | ||
Latest revision as of 10:23, 1 October 2010
 "
"