Team:UNIPV-Pavia/Calendar/September/settimana3
From 2010.igem.org
(→September, 15th) |
(→September, 16th) |
||
| (11 intermediate revisions not shown) | |||
| Line 88: | Line 88: | ||
and repeat | and repeat | ||
*I53: I32 (E-S) + I37 (E-X) | *I53: I32 (E-S) + I37 (E-X) | ||
| - | |||
| - | |||
| - | |||
---- | ---- | ||
| Line 113: | Line 110: | ||
==September, 15th== | ==September, 15th== | ||
| - | |||
| - | |||
| - | |||
Transformation of I 53, I59, I60, I61 ligations into ''E. coli'' DH5-alpha. They were plated on LB+Amp agar plates and let grow ON at 37°C. | Transformation of I 53, I59, I60, I61 ligations into ''E. coli'' DH5-alpha. They were plated on LB+Amp agar plates and let grow ON at 37°C. | ||
---- | ---- | ||
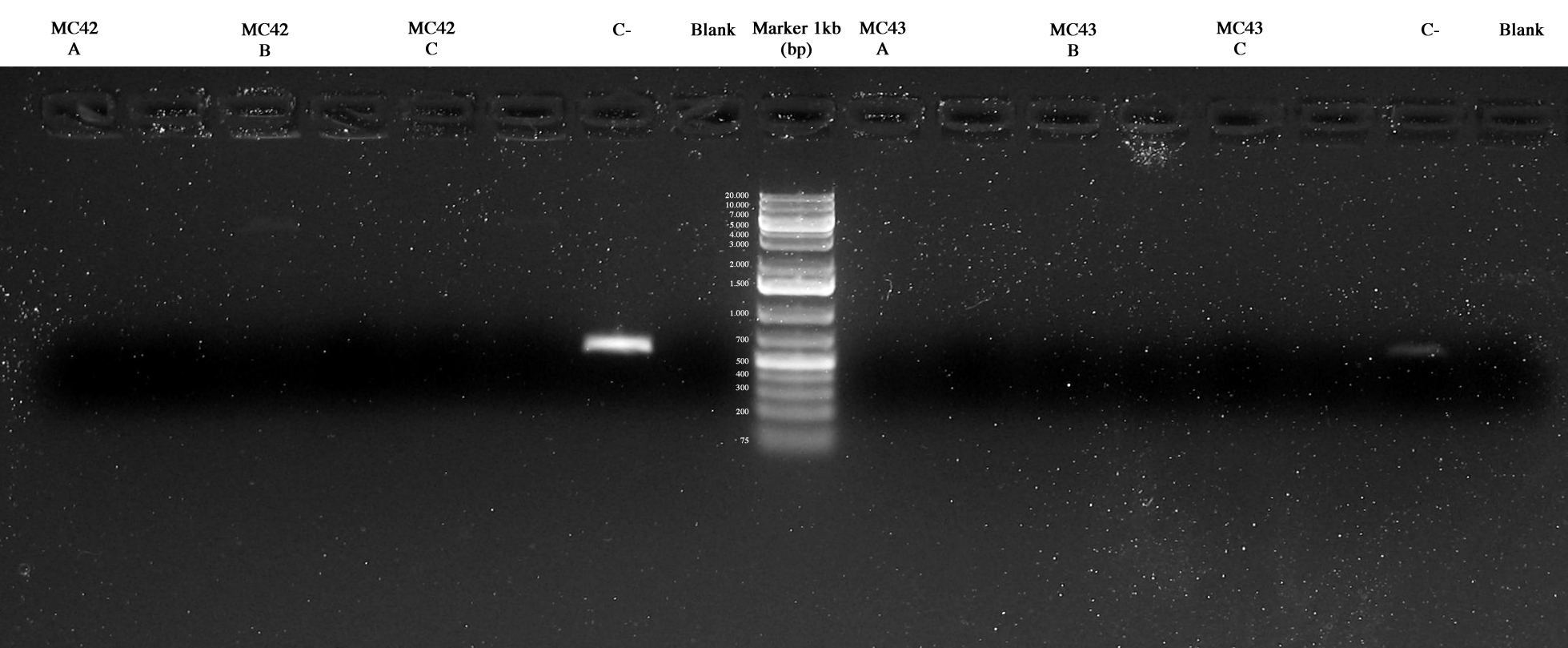
| - | 3 colonies were picked from each MG plates and screening PCR was performed also with MC42 and MC43 samples. Two methods with different DNA polymerases were used in order to identify best experimental conditions. The amplicons length (about 4Kb) probably was the reason of our problem in all the PCRs. | + | 3 colonies were picked from each MG plates and screening PCR was performed also with MC42 and MC43 samples. Two methods with different DNA polymerases were used in order to identify best experimental conditions (Taq polymerase and Pfx accuprime polymerase). The amplicons length (about 4Kb) probably was the reason of our problem in all the PCRs. |
| - | + | [[Image:UNIPV10_15_09_10_PCR_MARKER_MC42ABC_MG42ABC_Cneg_BLANK_MARKER_MG42ABC_MG43ABC_Cneg_BLANK(sopra Taq_sotto Pfx).jpg|thumb|250px|center| Screening PCR. In order: MC42ABC, MG42ABC, Cneg, BLANK, MG42ABC, MG43ABC, Cneg, BLANK (Taq samples over and Pfx samples under)]] | |
| - | + | ||
| - | + | ||
<div align="right"><small>[[#indice|^top]]</small></div> | <div align="right"><small>[[#indice|^top]]</small></div> | ||
| Line 153: | Line 145: | ||
---- | ---- | ||
| - | MC and MG cultures OD was referred to the lowest one in order to perform | + | MC and MG cultures OD was referred to the lowest one in order to perform Tecan Test (note that MG42-A didn't grow so we didn't perform test on this sample). Samples were then centrifuged: the resulting pellets were resuspended on 1 ml of PBS. |
---- | ---- | ||
| Line 188: | Line 180: | ||
|} | |} | ||
As you can see they are all positive (I58 was run a little longer to better separate bands). So we stocked and gel extracted I58c, I59b, I60a and only stocked I61a. | As you can see they are all positive (I58 was run a little longer to better separate bands). So we stocked and gel extracted I58c, I59b, I60a and only stocked I61a. | ||
| - | |||
| - | |||
| - | |||
<div align="right"><small>[[#indice|^top]]</small></div> | <div align="right"><small>[[#indice|^top]]</small></div> | ||
| Line 200: | Line 189: | ||
*I60 (E-S): 12.8 ng/ul | *I60 (E-S): 12.8 ng/ul | ||
DNA was than stored at -20°C. | DNA was than stored at -20°C. | ||
| - | |||
| - | |||
| - | |||
<div align="right"><small>[[#indice|^top]]</small></div> | <div align="right"><small>[[#indice|^top]]</small></div> | ||
| Line 208: | Line 194: | ||
==September, 19th== | ==September, 19th== | ||
Since there were no more colonies in I55 agar plate, we had to transform it again to perform screening through colony PCR. So I55 was transformed again into ''E. coli'' DH5-alpha and plated on LB+Amp agar plate, that was let grow ON at 37°C. | Since there were no more colonies in I55 agar plate, we had to transform it again to perform screening through colony PCR. So I55 was transformed again into ''E. coli'' DH5-alpha and plated on LB+Amp agar plate, that was let grow ON at 37°C. | ||
| - | |||
| - | |||
| - | |||
| - | |||
<div align="right"><small>[[#indice|^top]]</small></div> | <div align="right"><small>[[#indice|^top]]</small></div> | ||
Latest revision as of 11:16, 26 October 2010
|
|
||||||||||||||||||||||||||||||||||
|
|
|||||||||||||||||||||||||||||||||
 "
"