Team:St Andrews/project/modelling
From 2010.igem.org
| (17 intermediate revisions not shown) | |||
| Line 5: | Line 5: | ||
</html> | </html> | ||
| + | Hello and welcome to the University of St Andrews 2010 iGEM modelling pages. Here we present our work on the mathematical and computational modelling of the V.Cholerae bacterium and its quorum sensing system. What follows is an overall description of our work and our methodologies. For a more in depth description of our individual models please refer to our [[Team:St_Andrews/project/modelling/models| models page]] and to our [[Team:St_Andrews/project/modelling/downloads|downloads page]] where you can freely download and review our work | ||
| - | = | + | <html> |
| - | + | <center> | |
| + | <object width="480" height="385"><param name="movie" value="http://www.youtube.com/v/SZoUAoAFqmg?fs=1&hl=en_US&rel=0"></param><param name="allowFullScreen" value="true"></param><param name="allowscriptaccess" value="always"></param><embed src="http://www.youtube.com/v/SZoUAoAFqmg?fs=1&hl=en_US&rel=0" type="application/x-shockwave-flash" allowscriptaccess="always" allowfullscreen="true" width="480" height="385"></embed></object> | ||
| + | </center> | ||
| + | </html> | ||
| + | |||
| + | '''A short animation created to explain the basic biological principles behind our modelling''' | ||
| - | |||
| - | |||
= From the Biology... = | = From the Biology... = | ||
== Quorum sensing in E.Coli: The LuxR system == | == Quorum sensing in E.Coli: The LuxR system == | ||
| Line 28: | Line 32: | ||
| - | Converse to this, if CAI-1 attaches to CqsS or AI-2 to LuxPQ, the receptors change to phosphatases and remove phosphate groups from the LuxU, which removes them from LuxO. Thus the 4 sRNAs are not transcribed and there is no inhibition of HapR, such that virulence factors are no longer expressed. Instead, HapR promotes the | + | Converse to this, if CAI-1 attaches to CqsS or AI-2 to LuxPQ, the receptors change to phosphatases and remove phosphate groups from the LuxU, which removes them from LuxO. Thus the 4 sRNAs are not transcribed and there is no inhibition of HapR, such that virulence factors are no longer expressed. Instead, HapR promotes the production of a protein which cuts the V.Cholerae from the gut wall and allows it to pass out of the body in order to infect a different host. In this way, at low cell density the cholera causes infection in the host until reaching a critical density at which the behaviour changes and the bacteria exit the system. The internal dynamics of the system are more complex than this however. HapR attaches to its own promoter and thus is self-repressing, as is LuxO. HapR also promotes the transcription of the sRNAs and the sRNAs repress the transcription of LuxO. The system also has many unknown factors at play, however, and in combination with the lack of available rate constants makes it an ominous prospect to accurately model. |
== Components == | == Components == | ||
| Line 42: | Line 46: | ||
==What we used == | ==What we used == | ||
| - | In line with the nature of iGEM all of the software used in development of our models was Free | + | In line with the nature of iGEM all of the software used in development of our models was Free Software. We wish to thank each of the following communities for producing such high quality software: |
* [http://gcc.gnu.org/ GCC], used to compile our C++ programs | * [http://gcc.gnu.org/ GCC], used to compile our C++ programs | ||
Latest revision as of 02:26, 28 October 2010


Modelling Background
Hello and welcome to the University of St Andrews 2010 iGEM modelling pages. Here we present our work on the mathematical and computational modelling of the V.Cholerae bacterium and its quorum sensing system. What follows is an overall description of our work and our methodologies. For a more in depth description of our individual models please refer to our models page and to our downloads page where you can freely download and review our work
A short animation created to explain the basic biological principles behind our modelling
Contents |
From the Biology...
Quorum sensing in E.Coli: The LuxR system
The LuxR quorum sensing system is common among the vibrio family, and the lux system of the luminescent bacterium V.fischeri has been studied in great detail. This gave us a plentiful supply of scientific papers and review articles on which to base our understanding of the system. The LuxR system is underpinned by three quorum sensing molecules, those being the signalling molecule HSL; the HSL synthase LuxI and the transcriptional regulator LuxR. HSL may be generated in the cell by two means, either diffusion through the cell membrane from the external environment or being produced within the cell. Throughout the development of our models we considered a number of ways of trying to replicate this, and the details of these differenent techniques can be found in further sections. Whilst in the cell it may bind to LuxR, and two HSL molecules form a tetramer with two complementary LuxR molecules to form a complex which can then activate the lux operon. In the wild-type, this operon contains the genes coding for LuxI and those ultimately responsible for GFP production, with the luxR gene being located upstream and being constitutively produced. However, in our re-engineered operon the luxR gene lies downstream of the lux operon, thus its expression is also promoted. Hence once the promoter is activated, there is an increased production of LuxI, LuxR and also of GFP, which can therefore be used as an indicator of the promoter activity within the cell. Once transcription has been completed, the LuxI catalyses the production of more HSL from resources within the cell (which we have assumed exist in a sufficiently high concentration that they can be considered infinite). This may leave the cell due to a concentration gradient if the cell density in the medium is low, or otherwise will be used again in the loop, causing a positive feedback effect which will cause a high increase in the concentration of HSL. In this way the single bacterium can communicate with those surrounding it in order to evaluate whether it exists in sufficient numbers to overcome the immune system.
Quorum sensing in V.Cholerae: The LuxPQ system
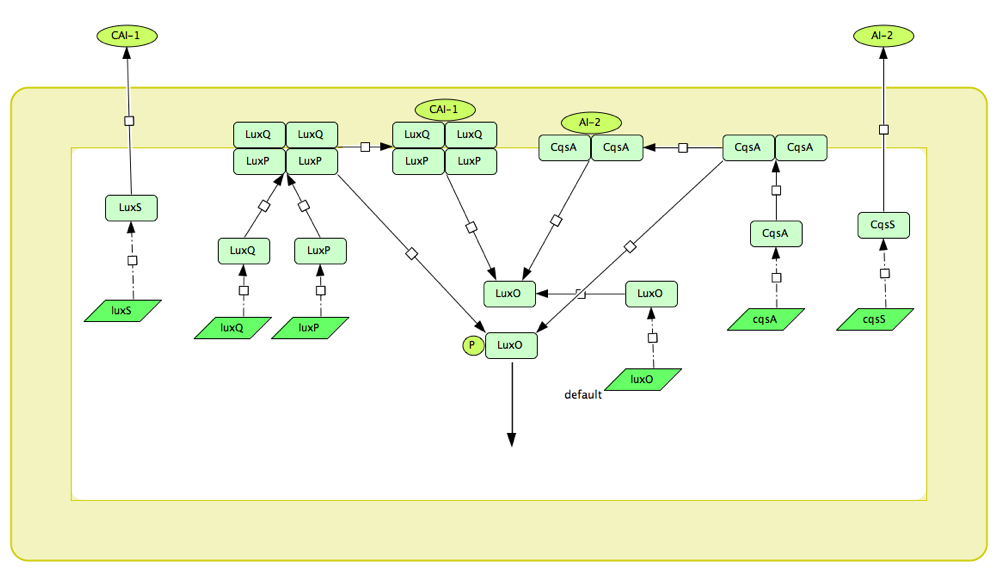
The V.cholerae quorum sensing circuit is considerably more complex than that of the LuxR system, consisting as it does of two circuits working in tandem. These are the CqsS and the LuxPQ circuits, which communicate with other bacterium and relay this information to a shared component, LuxU which ultimately controls the expression of virulence factors.
Figure 1: Schematic diagram of the LuxPQ and CqsS quorum sensing systems of V. cholerae
In the CqsS circuit, the CAI-1 (cholera autoinducer) molecule attaches to a dimerised complex of two CqsS molecules which exist in the inner membrane of the bacterium. When the CqsS dimer has not CAI-1 attached, it acts as a kinase and transfers phosphate groups down to LuxU which in turn phosphorylates LuxO. Similarly, another autoinducer molecule, AI-2 can attach to LuxPQ which is also in the inner membrane of the bacterium. When unattached the LuxPQ also phosphorylates LuxU in the same manner as CqsS and do the two systems are connected here. When phosphorylated, LuxO acts as a transcriptional regulator in the transcription of 4 sRNAs (small RNAs) which, in conjunction with the protein Hfq inhibit the production of what we have named the 'master regulator' HapR. HapR inhibits the expression of virulence factors, so at low cell density i.e. when CqsS and LuxPQ are kinases, HapR is repressed, and virulence factors are expressed.
Converse to this, if CAI-1 attaches to CqsS or AI-2 to LuxPQ, the receptors change to phosphatases and remove phosphate groups from the LuxU, which removes them from LuxO. Thus the 4 sRNAs are not transcribed and there is no inhibition of HapR, such that virulence factors are no longer expressed. Instead, HapR promotes the production of a protein which cuts the V.Cholerae from the gut wall and allows it to pass out of the body in order to infect a different host. In this way, at low cell density the cholera causes infection in the host until reaching a critical density at which the behaviour changes and the bacteria exit the system. The internal dynamics of the system are more complex than this however. HapR attaches to its own promoter and thus is self-repressing, as is LuxO. HapR also promotes the transcription of the sRNAs and the sRNAs repress the transcription of LuxO. The system also has many unknown factors at play, however, and in combination with the lack of available rate constants makes it an ominous prospect to accurately model.
Components
Our project can be divided into two main components: the engineering of a bistable switch into the LuxR quorum sensing system, and the integration of CqsA into the LuxR circuit. The aim of the modelling side of the project was to treat these two tasks independently and on their completion construct a combined model. However, this initial aim was proven to be almost impossible due to the lack of rate constants for the cholera system, which has only been understood in its full complexity relatively recently [1]. In order to reach a compromise, we have built a number of qualitatively accurate models for the bistable LuxR system, and outlined a framework of differential equations for the cholera system which are correct at the time of writing, and which requires more rate constants to be of further use. The work done on the bistable switch included an investigation into why exactly such a configuration of genes exhibits hysteretic behavior, and what parameters are of importance in determining the “level” of bistability of the system.
The purpose of our modelling is to accurately replicate the behaviour of the bistable switch in order to allow our E.coli to be tuned so that they switch off only when we are sure that all the V.cholerae have left the system and the host is free of infection.
To the Computer
Under the hood
All our models are based upon a series of ordinary differential equations (ODEs) each of which have been derived from either prior research papers or our own research. These equations are solved computationally via the Fourth Order Runge-Kutta Method (RK4) - the classical iterative method of approximating numerical ODEs. Seeking full control of the implementation of our model we decided against the use of mathematical packages and instead decided upon using a fully-fledged programming language to develop our models. Initially we decided upon GNU R, a programming language and environment for statistical computing. While R allowed for the rapid development of our initial models it was found that R lacked the fine grain control and the raw machine efficiency that we so required. A key problem that we encountered was that the majority of ODE solver libraries available for R were not in fact written in R, instead they were written in FORTRAN and C and the R component of the library meerly passed data to and from the external program. This was problematic as it did not allow us to access and modify the ODE solving algorithm which, in order to acquire the full set of data pertinent to any of or models and thus made future use of R unfeasible. Instead we turned to C++, a far lower level language than R hoping for greater power of design. Being a compiled language (with an efficient compiler) C++ programs proved far faster than their R counterparts and offered a far finer degree of control. Initially we developed our own RK4 based ODE solver and put to work developing all our models as C++ programs. Output of the each of the models was processed and graphed using the gnuplot graphing utility. The first step in developing a usable model of the behaviour of the bistable switch was to manufacture a working model of the LuxR quorum sensing circuit (See our animation for an overview of how the circuit works). Through discussions with the biologists, we were able to generate a circuit diagram which allowed us to better visualise the workings of the system and split it up into individual reactions which we could then assign differential equations to. Ongoing testing of the model, in conjunction with further insights into the biology led to tweaking and adjustments, leading to further models with added degrees of complexity and greater realism. The various models and their associated results can be found in the models section. Our ultimate goal is to create a model accurately predicting the results of adding samples of our engineered E.coli into the human gut in the event of a cholera infection.
What we used
In line with the nature of iGEM all of the software used in development of our models was Free Software. We wish to thank each of the following communities for producing such high quality software:
- [http://gcc.gnu.org/ GCC], used to compile our C++ programs
- [http://www.vim.org vim], a text editor used to write programs prior to compiling them
- [http://www.gnuplot.info gnuplot], command-line plotting software used to produce our plots
- [http://www.codeblocks.org/ Code::Blocks], a development environment used for creating our programs
Download
In a continuing commitment to Free Software, all of our models are released under the [http://www.gnu.org/licenses/gpl.html GNU General Public License Version 3]. If you would like to view the code for our software it is available in the downloads page.
Models
Full detail of our models can be found at "Models".
 "
"