Team:Washington/Gram Positive
From 2010.igem.org
(→Anthrax still poses a major threat) |
|||
| (171 intermediate revisions not shown) | |||
| Line 25: | Line 25: | ||
</html> | </html> | ||
<!---------------------------------------PAGE CONTENT GOES BELOW THIS----------------------------------------> | <!---------------------------------------PAGE CONTENT GOES BELOW THIS----------------------------------------> | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | = | + | |
| - | === The | + | =<big>'''Development of a Novel Anthrax Therapeutic'''</big>= |
| - | === | + | |
| + | [[Image:Washington_Gram_positive_banner2.jpg|800px|center]] | ||
| + | |||
| + | =='''Anthrax still poses a major threat'''== | ||
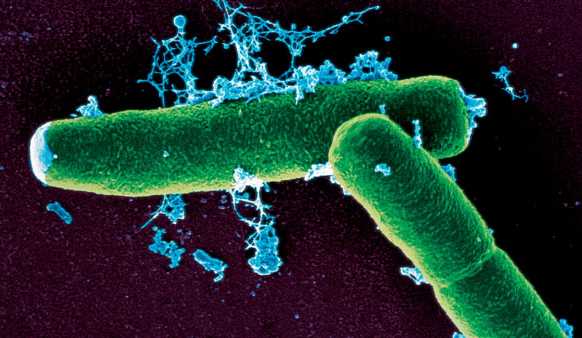
| + | [[Image:Washington_Bacillus_Anthracis.jpg|thumb|400px|right|''Bacillus anthracis'']] | ||
| + | |||
| + | Anthrax is a lethal disease caused by the bacterium ''Bacillus anthracis'', which can spread by ingestion, inhalation, or cutaneous lesion contact of spores. This disease is not contagious, cannot be transferred from an infected organism, though bacterial spores can be transported in a multitude of ways and will infect potential victims. [[#References | [1]]] | ||
| + | |||
| + | |||
| + | One of the primary reasons that Bacillus anthracis is such a deadly pathogen is its ability to evade the human immune system. The bacterium cloaks itself in an outer coat of poly-γ-D-glutamate (PDG) that confers antiphagocytic properties on the organism, and is essential for virulence [[#References | [2]]] | ||
| + | |||
| + | |||
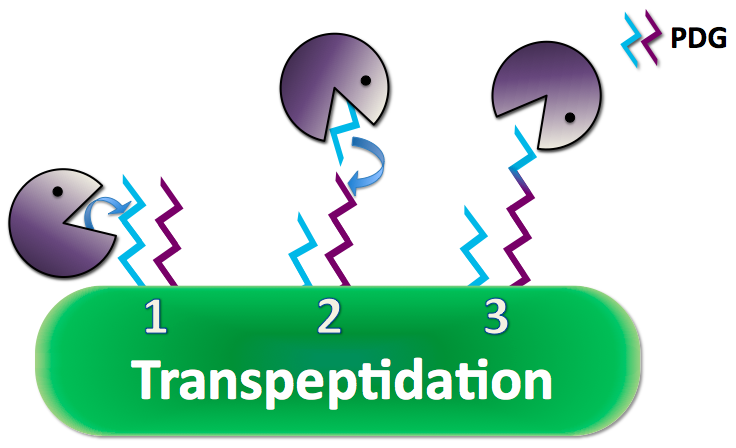
| + | The protective PDG coat is synthesized on the surface of ''B. anthracis'' by a series of enzymes. A key step of the synthesis is done by the enzyme CapD, which takes portions of a long strand of PDG, which is continuously secreted from the cell, and transfers it to the peptidoglycan layer through a transpeptidation reaction. It is important to note, however, that CapD has also been shown to have the capacity to catalyze hydrolysis of PDG during this process, but at a rate much slower than the competing transpeptidation reaction. [[#References | [3]]] | ||
| + | |||
| + | |||
| + | This small amount of hydrolase activity was co-opted in a therapeutic capacity in a guinea pig model [[#References | [4]]]. When wild type CapD was overexpressed, the small amount of hydrolase activity inherent in the many copies of the enzyme that were produced was sufficient to compromise the protective coating of the bacterium. The immune system was then able to clear the infection much more effectively. We therefore hoped to engineer a mutant CapD protein for which the propensity to carry out a protective transpeptidation reaction was reduced, and the propensity to hydrolyze the protective coat was increased. If a mutant CapD with hydrolytic, rather than transpeptidation activity (which we term CapH) could be engineered, a single, highly concentrated dose of the protein in an infected individual could prove a potent therapeutic. Alternatively, simply ablating the protective transpeptidase activity without compromising the pre-existing hydrolase activity would likely dramatically increase the potency of this protein as a potential therapeutic. | ||
| + | |||
| + | |||
| + | |||
| + | <span id=capd> | ||
| + | |||
| + | ==Capsule Depolymerase (CapD): Capsule Creator and Destroyer == | ||
| + | Anthrax creates a poly-γ-D-glutamate (PDG) capsule which prevents the immune system from recognizing it as a pathogen and performing phagocytosis to eliminate the threat. Researchs have shown that adding the enzyme capsule depolymerase to anthrax naturally, anthrax excretes a long strand of PDG and uses its CapD to cleave and anchor the PDG to its peptidoglycan, the outer coat of the cell membrane, as a protective capsule. CapD can also cut and release its capsule into small pieces that can interfere with the immune responses. | ||
| + | </span> | ||
| + | [[Image:WashingtonCapD_Trans.png|600px|center|]] | ||
| + | |||
| + | ==Potential Weapon against Anthrax== | ||
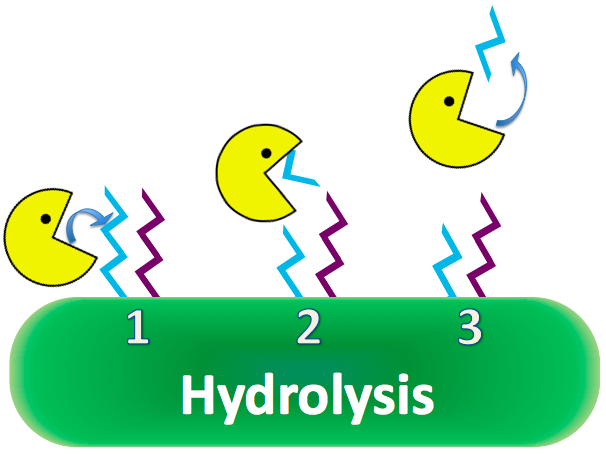
| + | Research based on a guinea pig model shows overexpression of CapD will destroy the capsule, removing anthrax's immunity to phagocytosis. | ||
| + | CapD is naturally a transpeptidase, favoring reactions with amino acids to cleave PDG. However, CapD would be invaluable as a hydrolase, reacting efficiently with water, because of high water content in the human bloodstream. If a mutant CapD could be engineered as an extremely efficient hydrolase, it is theorized that a single dose of concentrated CapD into the blood stream would easily decimate anthrax populations, nullifying its lethal properties. [[#References | [4]]] | ||
| + | [[Image:WashingtonCapDExplanation_Hydro.png|550px|center|]] | ||
| + | |||
| + | |||
| + | |||
| + | ==References== | ||
| + | 1. Lucey DR, Anthrax. In: Goldman L, Ausiello D, eds. Cecil Medicine. 23rd ed. Philadelphia, Pa: Saunders Elsevier; 2007:chap 317. | ||
| + | |||
| + | 2. Angelo Scorpio, Donald J. Chabot, William A. Day, David K. O'Brien, Nicholas J. Vietri, Yoshifumi Itoh, Mansour Mohamadzadeh, and Arthur M. Friedlander. Poly-γ-Glutamate Capsule-Degrading Enzyme Treatment Enhances Phagocytosis and Killing of Encapsulated Bacillus anthracis Antimicrobial Agents and Chemotherapy, January 2007, p. 215-222,Vol. 51, No. 1. PMCID: PMC1797643 | ||
| + | |||
| + | 3. Candela T, Fouet A. Bacillus anthracis CapD, belonging to the gamma-glutamyltranspeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Mol Microbiol. 2005 Aug;57(3):717-26. PMID: 16045616 | ||
| + | |||
| + | 4. Angelo Scorpio, Donald J Chabot, William A Day, Timothy A Hoover, and Arthur M Friedlander. Capsule depolymerase overexpression reduces Bacillus anthracis virulence Microbiology 2010 : mic.0.035857-0v1-mic.0.035857-0. PMID: 20110296 | ||
| + | |||
| + | |||
| + | |||
<!---------------------------------------PAGE CONTENT GOES ABOVE THIS----------------------------------------> | <!---------------------------------------PAGE CONTENT GOES ABOVE THIS----------------------------------------> | ||
<div style="text-align:center"> | <div style="text-align:center"> | ||
| - | '''[[Team:Washington/ | + | '''← [[Team:Washington/Gram Negative/Test|Testing the Gram(-) Therapeutic]]''' |
| + | | ||
| + | | ||
| + | | ||
| + | '''[[Team:Washington/Gram Positive/Design|Designing the Gram(+) Therapeutic]] →''' | ||
</div> | </div> | ||
{{Template:Team:Washington/Templates/Footer}} | {{Template:Team:Washington/Templates/Footer}} | ||
Latest revision as of 20:53, 27 October 2010
Development of a Novel Anthrax Therapeutic
Anthrax still poses a major threat
Anthrax is a lethal disease caused by the bacterium Bacillus anthracis, which can spread by ingestion, inhalation, or cutaneous lesion contact of spores. This disease is not contagious, cannot be transferred from an infected organism, though bacterial spores can be transported in a multitude of ways and will infect potential victims. [1]
One of the primary reasons that Bacillus anthracis is such a deadly pathogen is its ability to evade the human immune system. The bacterium cloaks itself in an outer coat of poly-γ-D-glutamate (PDG) that confers antiphagocytic properties on the organism, and is essential for virulence [2]
The protective PDG coat is synthesized on the surface of B. anthracis by a series of enzymes. A key step of the synthesis is done by the enzyme CapD, which takes portions of a long strand of PDG, which is continuously secreted from the cell, and transfers it to the peptidoglycan layer through a transpeptidation reaction. It is important to note, however, that CapD has also been shown to have the capacity to catalyze hydrolysis of PDG during this process, but at a rate much slower than the competing transpeptidation reaction. [3]
This small amount of hydrolase activity was co-opted in a therapeutic capacity in a guinea pig model [4]. When wild type CapD was overexpressed, the small amount of hydrolase activity inherent in the many copies of the enzyme that were produced was sufficient to compromise the protective coating of the bacterium. The immune system was then able to clear the infection much more effectively. We therefore hoped to engineer a mutant CapD protein for which the propensity to carry out a protective transpeptidation reaction was reduced, and the propensity to hydrolyze the protective coat was increased. If a mutant CapD with hydrolytic, rather than transpeptidation activity (which we term CapH) could be engineered, a single, highly concentrated dose of the protein in an infected individual could prove a potent therapeutic. Alternatively, simply ablating the protective transpeptidase activity without compromising the pre-existing hydrolase activity would likely dramatically increase the potency of this protein as a potential therapeutic.
Capsule Depolymerase (CapD): Capsule Creator and Destroyer
Anthrax creates a poly-γ-D-glutamate (PDG) capsule which prevents the immune system from recognizing it as a pathogen and performing phagocytosis to eliminate the threat. Researchs have shown that adding the enzyme capsule depolymerase to anthrax naturally, anthrax excretes a long strand of PDG and uses its CapD to cleave and anchor the PDG to its peptidoglycan, the outer coat of the cell membrane, as a protective capsule. CapD can also cut and release its capsule into small pieces that can interfere with the immune responses.
Potential Weapon against Anthrax
Research based on a guinea pig model shows overexpression of CapD will destroy the capsule, removing anthrax's immunity to phagocytosis. CapD is naturally a transpeptidase, favoring reactions with amino acids to cleave PDG. However, CapD would be invaluable as a hydrolase, reacting efficiently with water, because of high water content in the human bloodstream. If a mutant CapD could be engineered as an extremely efficient hydrolase, it is theorized that a single dose of concentrated CapD into the blood stream would easily decimate anthrax populations, nullifying its lethal properties. [4]
References
1. Lucey DR, Anthrax. In: Goldman L, Ausiello D, eds. Cecil Medicine. 23rd ed. Philadelphia, Pa: Saunders Elsevier; 2007:chap 317.
2. Angelo Scorpio, Donald J. Chabot, William A. Day, David K. O'Brien, Nicholas J. Vietri, Yoshifumi Itoh, Mansour Mohamadzadeh, and Arthur M. Friedlander. Poly-γ-Glutamate Capsule-Degrading Enzyme Treatment Enhances Phagocytosis and Killing of Encapsulated Bacillus anthracis Antimicrobial Agents and Chemotherapy, January 2007, p. 215-222,Vol. 51, No. 1. PMCID: PMC1797643
3. Candela T, Fouet A. Bacillus anthracis CapD, belonging to the gamma-glutamyltranspeptidase family, is required for the covalent anchoring of capsule to peptidoglycan. Mol Microbiol. 2005 Aug;57(3):717-26. PMID: 16045616
4. Angelo Scorpio, Donald J Chabot, William A Day, Timothy A Hoover, and Arthur M Friedlander. Capsule depolymerase overexpression reduces Bacillus anthracis virulence Microbiology 2010 : mic.0.035857-0v1-mic.0.035857-0. PMID: 20110296
 "
"