|
|
| (6 intermediate revisions not shown) |
| Line 15: |
Line 15: |
| | 2 uL PCR product of B0015 (no. 43 white) was used as template for each PCR reaction.3 PCR reactions were prepared.<br> | | 2 uL PCR product of B0015 (no. 43 white) was used as template for each PCR reaction.3 PCR reactions were prepared.<br> |
| | Premix for 4 PCR reactions:<br> | | Premix for 4 PCR reactions:<br> |
| - | <table style="text-align: left; width: 100%;" border="1" | + | <table style="text-align: left; width: 300px;" border="1" |
| | cellpadding="2" cellspacing="2"> | | cellpadding="2" cellspacing="2"> |
| | <tr> | | <tr> |
| Line 45: |
Line 45: |
| | 48uL premix is distrubuted into each PCR tube. PCR tubes are marked B0015.A-C<br> | | 48uL premix is distrubuted into each PCR tube. PCR tubes are marked B0015.A-C<br> |
| | PCR program:<br> | | PCR program:<br> |
| - | <table style="text-align: left; width: 100%;" border="1" | + | <table style="text-align: left; width: 300px;" border="1" |
| | cellpadding="2" cellspacing="2"> | | cellpadding="2" cellspacing="2"> |
| | <tr> | | <tr> |
| Line 93: |
Line 93: |
| | Start date: 10/8<br> | | Start date: 10/8<br> |
| | Methods: ON, sonication, UV-vis spectroscopy<br> | | Methods: ON, sonication, UV-vis spectroscopy<br> |
| - | Protocols: CC.1.1[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1] | + | Protocols: [https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP.1.1] |
| | ==== Colony PCR on transformants using ninaB fwd and rw primers ==== | | ==== Colony PCR on transformants using ninaB fwd and rw primers ==== |
| | Date: 10/8<br> | | Date: 10/8<br> |
| Line 101: |
Line 101: |
| | <br><br> | | <br><br> |
| | Notes:<br> | | Notes:<br> |
| - | ON was made from 4 colonies:<br> | + | Over night (ON) cultures were grown from 4 colonies, until the following OD’s were obtained: |
| - | Top 10 - on insert -> OD = 0,008 (100 x diluted)<br>
| + | |
| - | Top 10 - with K274210 biobrick insert -> OD = 0,011 (100 x diluted)<br>
| + | |
| - | MG1655 - on insert -> OD = 0,020 (100 x diluted)<br>
| + | |
| - | Mg1655 - with K274210 biobrick insert -> OD = 0,017 (100 x diluted)<br>
| + | |
| - | ON was made in 110 ml LB media. colonies with K274210 biobrick insert was grown in media containing ampicillin.<br>
| + | |
| - | alle the ON cultures was grown for 20 hours at 37 °C.<br>
| + | |
| - | After 16 hours, ml of the ON colonies was transferred in to 110 LB media and grown for 4 hours to the exponential phase:<br>
| + | |
| - | Top 10 - on insert -> OD = 0,049 (100 x diluted)<br>
| + | |
| - | Top 10 - with K274210 biobrick insert -> OD = 0,044 (100 x diluted)<br>
| + | |
| - | MG1655 - on insert -> OD = 0,007 (100 x diluted)<br>
| + | |
| - | Mg1655 - with K274210 biobrick insert -> OD = 0,009 (100 x diluted)<br>
| + | |
| - | Colonies with K274210 biobrick insert was grown in media containing ampicillin.<br>
| + | |
| | | | |
| - | ==== Harvesting, sonication and UV-vis spektroscopy ==== | + | Top 10 - no insert OD = 0,008 (100 x diluted)<br> |
| - | Date: 11/8<br>
| + | Top 10 - with K274210 insert OD = 0,011 (100 x diluted)<br> |
| - | Done by: Christian & Tommy<br>
| + | MG1655 - no insert OD = 0,020 (100 x diluted)<br> |
| - | Methods: Cell harvesting, sonication and UV-vis messurements<br>
| + | Mg1655 - with K274210 insert OD = 0,017 (100 x diluted)<br> |
| - | protocos: none<br>
| + | |
| - | <br> | + | ON cultures were grown in 110 ml LB media. Colonies with K274210 insert were grown in LB media containing ampicillin. |
| - | Notes:<br>
| + | All ON cultures were grown for 20 hours at 37 °C. |
| - | 100 mL Cell cultures was centrifuged for 5 min at 14000 RPM. the supernantant was discarded and cells were resuspended in 5 mL acetone (99,9%), except the top 10 with the K274210 biobrick insert wicht was resuspended in 10 mL acetone (suorce of error.). The resuspended cells were sonicated for 5 min. the samples were spun down and the supernantant were transferrede to new tubes, cell bebri was discarded.<br> | + | After 16 hours, 10 ml of the ON cultures were transferred into 110 ml LB media and grown for 4 hours to reach the exponential phase, where the following OD’s were obtained: |
| - | A standart curve was made from pure Beta-carotene,<br> | + | |
| - | The samples and the standarts were first measured at a fixed wavelengt of 456 nm. | + | Top 10 - no insert OD = 0,049 (100 x diluted)<br> |
| - | The standarts:<br>
| + | Top 10 - with K274210 insert OD = 0,044 (100 x diluted)<br> |
| - | <table style="text-align: left; width: 100%;" border="1" | + | MG1655 - no insert OD = 0,007 (100 x diluted)<br> |
| | + | Mg1655 - with K274210 insert OD = 0,009 (100 x diluted)<br> |
| | + | |
| | + | Cultures with K274210 insert were grown in media containing ampicillin. |
| | + | 100 mL cell culture were centrifuged for 5 min at 14000 RPM. The supernatant was discarded and cells were resuspended in 5 mL acetone (99,9%), except the Top 10 E. coli with the K274210 insert, which was resuspended in 10 mL acetone (source of error). The resuspended cells were sonicated for 5 min. Samples were spun down, the supernatant was transferred to new tubes, and cell debris was discarded. A standard curve was made from pure beta-carotene.<br> |
| | + | |
| | + | The samples at the OD’s seen above as well as solutions of pure beta-carotene with known concentrations were measured at a fixed wavelength of 456 nm. |
| | + | Known concentrations and their absorbances: |
| | + | <table style="text-align: left; width: 300px;" border="1" |
| | cellpadding="2" cellspacing="2"> | | cellpadding="2" cellspacing="2"> |
| | <tr> | | <tr> |
| Line 177: |
Line 173: |
| | </tr> | | </tr> |
| | </table> | | </table> |
| - | The samples:<br> | + | The samples and their absorbances:<br> |
| - | <table style="text-align: left; width: 100%;" border="1" | + | <table style="text-align: left; width: 300px;" border="1" |
| | cellpadding="2" cellspacing="2"> | | cellpadding="2" cellspacing="2"> |
| | <tr> | | <tr> |
| Line 206: |
Line 202: |
| | </tr> | | </tr> |
| | </table> | | </table> |
| - | After this the standarts and samples were measured on a UV-vis spetrometer. The stationary phases expersion of beta-carotene by wild strain MG1655 E. coli and top 10 E. coli cells with standarts solutions of beta-carotene, the graf is shown below<br>
| + | UV-VIS absorbance spectra of the known solutions were obtained, as well as spectra of the samples at the ODs shown above. The spectra of Top 10 and MG1655 in the stationary phase as well as selected spectra of known solutions are shown below:<br> |
| - | [[Image:Team-SDU-denmarkBeta-carotene.jpg |400px]] | + | [[Image:Team-SDU-denmarkBeta-carotene.jpg |500px]] |
| | === PCR of NinaB (again) === | | === PCR of NinaB (again) === |
| | Start date: 13/8<br> | | Start date: 13/8<br> |
| | Methods: Restriction digest, PCR, gel<br> | | Methods: Restriction digest, PCR, gel<br> |
| - | Protocols: RD1.1 [https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1], CC.1.1[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1] | + | Protocols: [https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1 RD1.1], .1[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP.1] |
| | ==== Colony PCR on transformants using ninaB fwd and rw primers ==== | | ==== Colony PCR on transformants using ninaB fwd and rw primers ==== |
| | Date: 13/8<br> | | Date: 13/8<br> |
| Line 220: |
Line 216: |
| | Notes:<br> | | Notes:<br> |
| | Restirction digest was performed with EcoRI according to protocol. (gel was run on protocol).<br> | | Restirction digest was performed with EcoRI according to protocol. (gel was run on protocol).<br> |
| - | [[Image:Team-sdu-denmark-Minipreb_product_of_POT2_with_NinaB_1,5_gel_red_loader_-3.jpg |400 px ]] | + | [[Image:Team-sdu-denmark-Minipreb_product_of_POT2_with_NinaB_1,5_gel_red_loader_-3.jpg |300 px ]] <br> |
| | PCR was run on the product (No gel purification).<br> | | PCR was run on the product (No gel purification).<br> |
| | The following PCR program was used: | | The following PCR program was used: |
| - | <table style="text-align: left; width: 100%;" border="1" | + | <table style="text-align: left; width: 300px;" border="1" |
| | cellpadding="2" cellspacing="2"> | | cellpadding="2" cellspacing="2"> |
| | <tr> | | <tr> |
| Line 277: |
Line 273: |
| | </table> | | </table> |
| | gel was run on the PCR product.<br> | | gel was run on the PCR product.<br> |
| - | [[Image:Team-sdu-denmark-Minipreb_product_of_POT2_with_NinaB_1,5_gel_red_loader_-3.jpg |400px ]] | + | [[Image:Team-sdu-denmark-Minipreb_product_of_POT2_with_NinaB_1,5_gel_red_loader_-3.jpg |300px ]] |
| | + | |
| | === Miniprep of POT2 with NinaB insert === | | === Miniprep of POT2 with NinaB insert === |
| | Start date: 13/8<br> | | Start date: 13/8<br> |
| | Methods: Miniprep, Restriction digest, gel<br> | | Methods: Miniprep, Restriction digest, gel<br> |
| - | Protocols: MP1.2 [https://2010.igem.org/Team:SDU-Denmark/protocols#MP1.2], RD1.1 [https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1]. | + | Protocols: [https://2010.igem.org/Team:SDU-Denmark/protocols#MP1.2 MP1.2], [https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1 RD1.1]. |
| - | ==== ON of Top 10 E. coli cells with POT2 with NinaB ====
| + | |
| - | Date: 16/8<br>
| + | |
| - | Done by: Marie & Tommy<br>
| + | |
| - | Methods:
| + | |
| - | protocos:
| + | |
| - | <br><br>
| + | |
| - | Notes:<br>
| + | |
| - | 100 mL ON culture was made, cells were grown at 37 C in LB media with Chloramphenicol.
| + | |
| - | ==== ON of Top 10 E. coli cells with POT2 with NinaB ====
| + | |
| - | Date: 17/8<br>
| + | |
| - | Done by: Marie & Tommy<br>
| + | |
| - | Methods: Miniprep, Restriction digest, gel<br>
| + | |
| - | Protocols: MP1.2 [https://2010.igem.org/Team:SDU-Denmark/protocols#MP1.2], RD1.1 [https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1].
| + | |
| - | <br><br>
| + | |
| - | Notes:<br>
| + | |
| - | 50 mL of elution buffer was used. DNA concentrations after pooling were measured on NanoDrop to 206,2 ng/microL.
| + | |
| - | gel was run on produckt, showing recent miniprep compared to old miniprep produckt, showing uneven lengths. subsequent was performed.<br>
| + | |
| - | [[Image:Team-sdu-denmark-Minipreb_product_of_POT2_with_NinaB_1,5_gel_red_loader__-4.jpg | 400px ]]
| + | |
| - | ==== Restriction digest on miniprep produckt (w. EcoRI) ====
| + | |
| - | Date: 17/8<br>
| + | |
| - | Done by: Marie & Tommy<br>
| + | |
| - | Methods: Restriction digest, gel<br>
| + | |
| - | Protocols: MP1.2 [https://2010.igem.org/Team:SDU-Denmark/protocols#MP1.2], gel.
| + | |
| - | <br><br>
| + | |
| - | Notes:<br>
| + | |
| - | Due to the lack of old sample, restriction digest was performed using only 3,5 microL of miniprep produckt. 1,5 microL of H2O was added insted. EcoRI was used<br>
| + | |
| - | The digest mix was incubated for 10 min at 37 C. A gel was run showing uncut new, cut new, uncut old and cut old miniprep product.<br>
| + | |
| - | [[Image:Team-sdu-denmark-Minipreb_product_of_POT2_with_NinaB_1,5_gel_red_loader__-5.jpg |400px ]]
| + | |
Lab notes (8/9 - 8/15)
Group: Photosensor
pfu PCR of B0015 and PCR purification
date: 17/8
Protocols: CP1.1 and GFX easy protocol
Notes:
2 uL PCR product of B0015 (no. 43 white) was used as template for each PCR reaction.3 PCR reactions were prepared.
Premix for 4 PCR reactions:
| 25uL |
pfu bf. + MgSO4 |
| 7,5uL |
dNTP's |
| 7,5uL |
VF2 |
| 7,5uL |
VR |
| 190uL |
H20 |
| 2uL |
pfu Polymerase enzyme |
48uL premix is distrubuted into each PCR tube. PCR tubes are marked B0015.A-C
PCR program:
| Start |
95C |
3min |
| Denaturating |
95C |
2min |
| Annealing |
55C |
30s |
| Elongation |
72C |
45s |
| Go to |
2 |
29x |
| End |
72C |
2min |
| Hold |
4C |
|
5uL of PCR sample is loaded onto a 2% agarose gel. Generuler 100bp DNA ladder (blue) is used as marker.
Results:
Analysis:
PCR product is OK and B0015 DNA is purified from the PCR product according to protocol. DNA is eluted in 20uL H2O. Purified samples are stored in the jumblebox (hotchpotch), marked as B0015-1 and B0015-2.
Group: Retinal
Characterization of Beta-carotene
Start date: 10/8
Methods: ON, sonication, UV-vis spectroscopy
Protocols: CP.1.1
Colony PCR on transformants using ninaB fwd and rw primers
Date: 10/8
Done by: Christian & Tommy
Methods: ON
protocos:
Notes:
Over night (ON) cultures were grown from 4 colonies, until the following OD’s were obtained:
Top 10 - no insert OD = 0,008 (100 x diluted)
Top 10 - with K274210 insert OD = 0,011 (100 x diluted)
MG1655 - no insert OD = 0,020 (100 x diluted)
Mg1655 - with K274210 insert OD = 0,017 (100 x diluted)
ON cultures were grown in 110 ml LB media. Colonies with K274210 insert were grown in LB media containing ampicillin.
All ON cultures were grown for 20 hours at 37 °C.
After 16 hours, 10 ml of the ON cultures were transferred into 110 ml LB media and grown for 4 hours to reach the exponential phase, where the following OD’s were obtained:
Top 10 - no insert OD = 0,049 (100 x diluted)
Top 10 - with K274210 insert OD = 0,044 (100 x diluted)
MG1655 - no insert OD = 0,007 (100 x diluted)
Mg1655 - with K274210 insert OD = 0,009 (100 x diluted)
Cultures with K274210 insert were grown in media containing ampicillin.
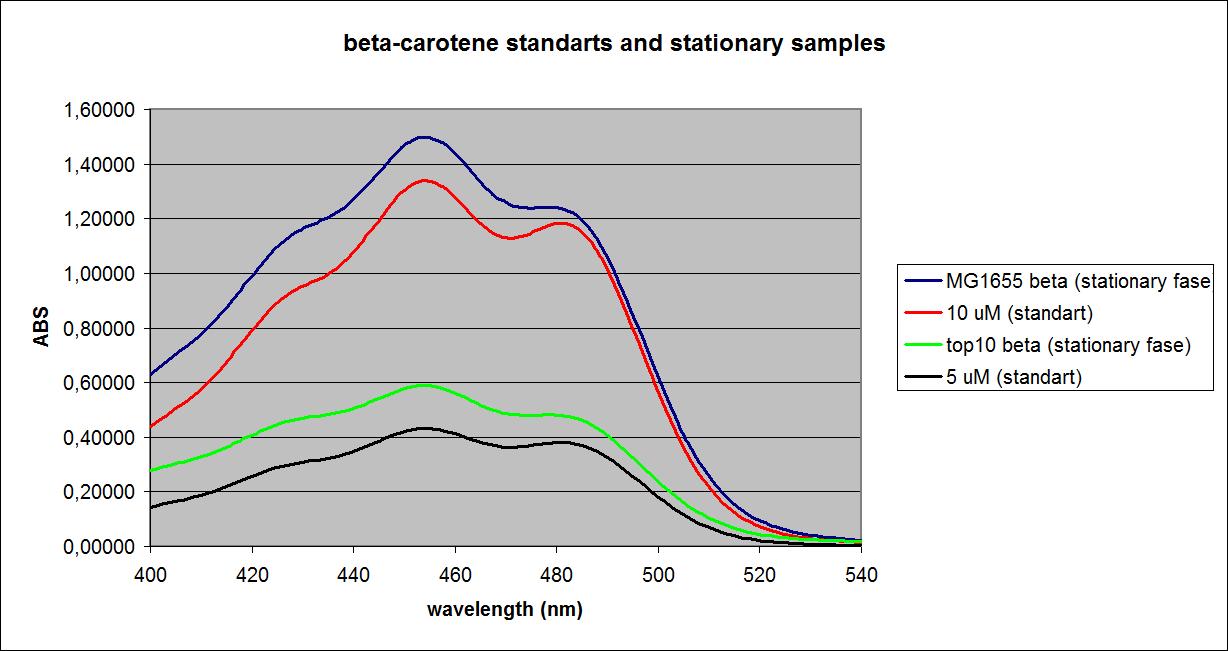
100 mL cell culture were centrifuged for 5 min at 14000 RPM. The supernatant was discarded and cells were resuspended in 5 mL acetone (99,9%), except the Top 10 E. coli with the K274210 insert, which was resuspended in 10 mL acetone (source of error). The resuspended cells were sonicated for 5 min. Samples were spun down, the supernatant was transferred to new tubes, and cell debris was discarded. A standard curve was made from pure beta-carotene.
The samples at the OD’s seen above as well as solutions of pure beta-carotene with known concentrations were measured at a fixed wavelength of 456 nm.
Known concentrations and their absorbances:
| Concentration |
Absorbance |
| 1 mM |
4,000 |
| 100 µM |
2,260 |
| 50 µM |
4,000 |
| 25 µM |
0,155 |
| 10 µM |
0,893 |
| 5 µM |
0,440 |
| 1 µM |
0,075 |
| 100 nM |
0,015 |
| 10 nM |
0,038 |
| 1 nM |
0,005 |
| 100 pM |
0,024 |
The samples and their absorbances:
|
Top 10 cells (Absorbance) |
MG1655 E. coli mutant (Absorbance) |
| Stationary phase control |
0,034 |
0,024 |
| Stationary phase with K274210 biobrick insert |
0,319 |
1,549 |
| Expotential phase control |
0,020 |
0,024 |
| Expotential phase with K274210 biobrick insert |
0,034 |
0,033 |
UV-VIS absorbance spectra of the known solutions were obtained, as well as spectra of the samples at the ODs shown above. The spectra of Top 10 and MG1655 in the stationary phase as well as selected spectra of known solutions are shown below:
PCR of NinaB (again)
Start date: 13/8
Methods: Restriction digest, PCR, gel
Protocols: RD1.1, .1CP.1
Colony PCR on transformants using ninaB fwd and rw primers
Date: 13/8
Done by: Marie & Tommy
Methods: Restriction digest, PCR, gel
protocos: RD1.1, CC.1.1
Notes:
Restirction digest was performed with EcoRI according to protocol. (gel was run on protocol).
PCR was run on the product (No gel purification).
The following PCR program was used:
| PCR |
Temp. (C) |
Time |
| Start |
95 |
2 min |
| Denaturation |
95 |
45 sec |
| Annealing |
49 |
30 sec |
| Elongation |
72 |
4 min |
| Denaturing |
95 |
45 sec |
| Annealing |
69 |
30 sec |
| Elongation |
72 |
4 min |
| End |
72 |
5 min |
| Hold |
4 |
indef. |
gel was run on the PCR product.

Miniprep of POT2 with NinaB insert
Start date: 13/8
Methods: Miniprep, Restriction digest, gel
Protocols:
MP1.2,
RD1.1.
 "
"

