Team:Newcastle/14 June 2010
From 2010.igem.org
Shethharsh08 (Talk | contribs) |
Shethharsh08 (Talk | contribs) (→Streak plating) |
||
| (35 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Team:Newcastle/mainbanner}} | {{Team:Newcastle/mainbanner}} | ||
| - | + | This is the '''FIRST DAY''' of the iGEM lab training. We were given several important tips by Dr. Wendy Smith to begin with, and then throughout the day, we were familiarized with basic lab techniques, e.g. how to use pipettes. | |
| - | This is the ''' | + | |
| + | =Wet Lab techniques practice= | ||
| + | |||
| + | ==Aims== | ||
| + | The aim of today's Lab training session was to practice aseptic techniques and wet lab techniques such as streak plating and broth culture preparation. | ||
| + | |||
| + | ==Materials Required== | ||
| + | |||
| + | '''For today's session we needed''': | ||
| + | |||
| + | * Agar plates | ||
| + | * Pipettes | ||
| + | * Wire loops | ||
| + | * ''E.coli'' (from a colony) | ||
| + | * LB broth | ||
| + | * Burnsen burner | ||
| + | * Conical flasks | ||
| + | * Orbital shaker | ||
| + | |||
| + | ==List of techniques== | ||
| + | #Used electronic balance and made LB broth. | ||
| + | #Prepared broth culture | ||
| + | #Familiarized with using pipettes of different volumes | ||
| + | #Mini-Prep introduction for Tuesday. | ||
| + | |||
| + | The biobrick '''BBa_J04450''''s prefix and suffix were identified from the parts registry. They are EcoRI and PstI respectively. | ||
==Tips== | ==Tips== | ||
| - | * | + | * Use aseptic technique: Treat everything as a pathogen! |
| - | + | * Work around burnsen burner. | |
| - | + | * Use a control for every experiment. | |
| - | + | * Clean the bench at the end of the day. | |
| - | *For individual colony | + | * Heat the flame loop from the middle to the tip till it becomes red hot. |
| - | *Wear gloves, especially when working with DNA, | + | * Keep plates upside down so that condensation does not damage the colonies. |
| + | * Keep the lids of the plates off for as short time as possible. | ||
| + | * Loosen all the tops off the vessels before you start. | ||
| + | * Flame tops of the bottles lightly so as to reduce the chances of contamination. | ||
| + | * Colony plates should be labelled (name of culture, antibiotics used, our initials and date) at the base and inverted in order to prevent condensation. | ||
| + | * For individual colony selection, steak across a few times in different directions in order to dilute the number of colonies per unit area. | ||
| + | * Wear gloves, especially when working with DNA, PCR etc. However, never wear gloves when working near a flame. | ||
| + | |||
| + | ==Streak plating== | ||
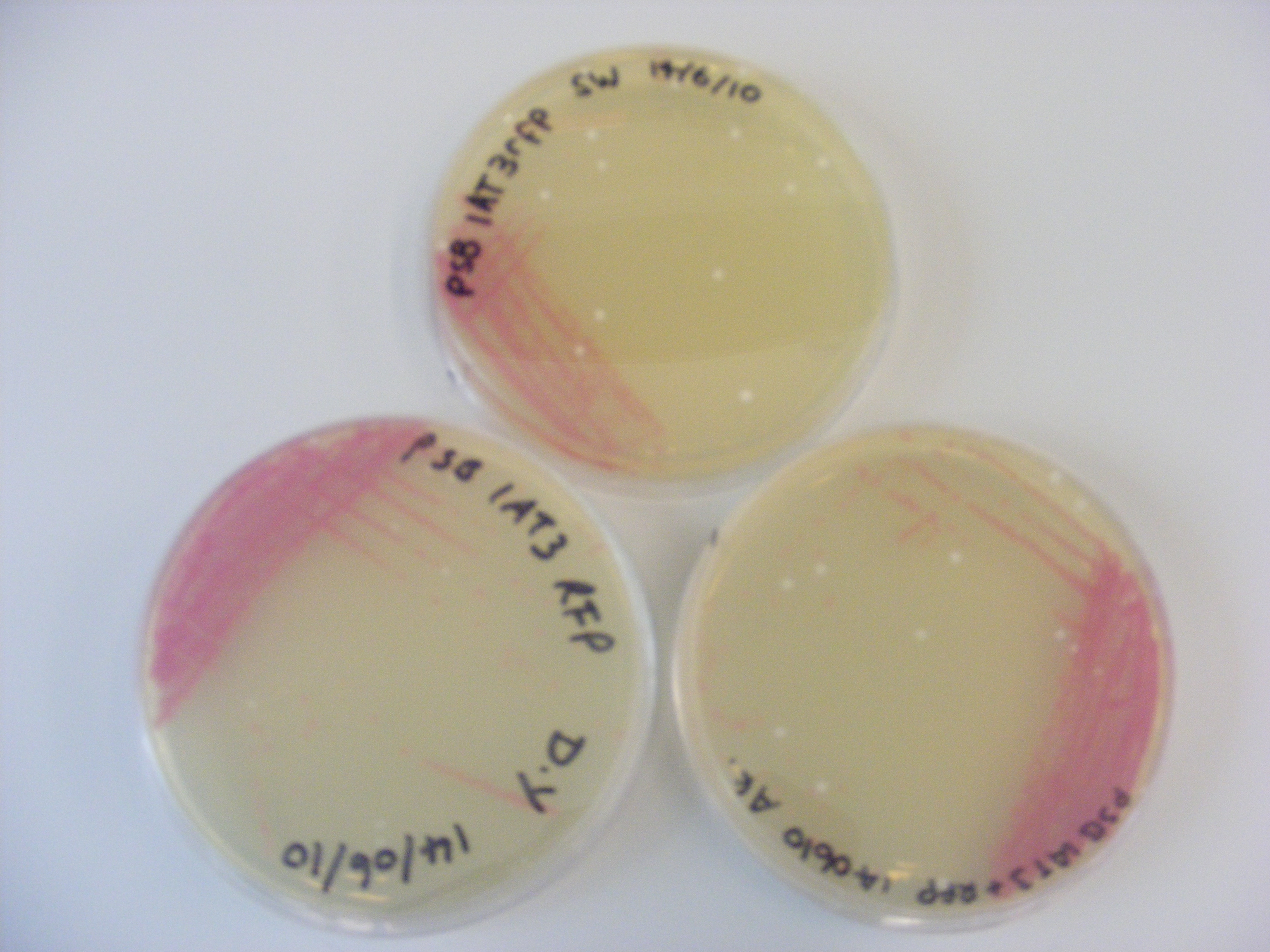
| + | [[Image:Newcastle_streak1.JPG|400px|Streak plating of ''E. coli'' DH5α cells]] | ||
| + | |||
| + | '''Picture 1''': Picture showing streak plate of ''E. coli'' DH5α cells containing plasmid pSB1AT3. The pink colouration on the plate is due to the ''rfp'' in the plasmid. | ||
| + | |||
| + | ==Setting up of the culture for plasmid miniprep extraction== | ||
| + | |||
| + | Refer to the protocol list for preparation of the overnight culture of ''E. coli'' DH5α cells containing pSB1AT3 plasmid: [[Team:Newcastle/Growing an overnight cultures| Growing an overnight culture]]. | ||
| + | Tomorrow we will harvest the plasmid DNA. | ||
| - | == | + | ==Conclusion== |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | We learnt aseptic techniques and some basic wet lab techniques. As you can see in the '''Picture 1''' shown above, our streak plates worked. | |
{{Team:Newcastle/footer}} | {{Team:Newcastle/footer}} | ||
Latest revision as of 18:02, 21 October 2010

| |||||||||||||
| |||||||||||||
This is the FIRST DAY of the iGEM lab training. We were given several important tips by Dr. Wendy Smith to begin with, and then throughout the day, we were familiarized with basic lab techniques, e.g. how to use pipettes.
Contents |
Wet Lab techniques practice
Aims
The aim of today's Lab training session was to practice aseptic techniques and wet lab techniques such as streak plating and broth culture preparation.
Materials Required
For today's session we needed:
- Agar plates
- Pipettes
- Wire loops
- E.coli (from a colony)
- LB broth
- Burnsen burner
- Conical flasks
- Orbital shaker
List of techniques
- Used electronic balance and made LB broth.
- Prepared broth culture
- Familiarized with using pipettes of different volumes
- Mini-Prep introduction for Tuesday.
The biobrick BBa_J04450's prefix and suffix were identified from the parts registry. They are EcoRI and PstI respectively.
Tips
- Use aseptic technique: Treat everything as a pathogen!
- Work around burnsen burner.
- Use a control for every experiment.
- Clean the bench at the end of the day.
- Heat the flame loop from the middle to the tip till it becomes red hot.
- Keep plates upside down so that condensation does not damage the colonies.
- Keep the lids of the plates off for as short time as possible.
- Loosen all the tops off the vessels before you start.
- Flame tops of the bottles lightly so as to reduce the chances of contamination.
- Colony plates should be labelled (name of culture, antibiotics used, our initials and date) at the base and inverted in order to prevent condensation.
- For individual colony selection, steak across a few times in different directions in order to dilute the number of colonies per unit area.
- Wear gloves, especially when working with DNA, PCR etc. However, never wear gloves when working near a flame.
Streak plating
Picture 1: Picture showing streak plate of E. coli DH5α cells containing plasmid pSB1AT3. The pink colouration on the plate is due to the rfp in the plasmid.
Setting up of the culture for plasmid miniprep extraction
Refer to the protocol list for preparation of the overnight culture of E. coli DH5α cells containing pSB1AT3 plasmid: Growing an overnight culture.
Tomorrow we will harvest the plasmid DNA.
Conclusion
We learnt aseptic techniques and some basic wet lab techniques. As you can see in the Picture 1 shown above, our streak plates worked.
 
|
 "
"