Team:SDU-Denmark/labnotes2
From 2010.igem.org
(Difference between revisions)
(→Purification of pSB1A2 containing J13002) |
(→Group: Retinal) |
||
| (55 intermediate revisions not shown) | |||
| Line 176: | Line 176: | ||
</html> | </html> | ||
| - | ''Results:'' A gel, containing the PCR product was run, using [http://www.fermentas.de/product_info.php?info=p1110 GeneRuler(TM) DNA Ladder Mix, ready-to-use (#SM0333)] loaded in lane one | + | ''Results:'' A gel, containing the PCR product was run, using [http://www.fermentas.de/product_info.php?info=p1110 GeneRuler(TM) DNA Ladder Mix, ready-to-use (#SM0333)] loaded in lane one, lane 2 contains sample run at 56,1 degrees celcius and in lane 3 sample run at 64,5 degrees celcius is found.<br><br> |
[[Image:Annealing of the two mutated FlhDC strands (sheila).jpg|300px]]<br> | [[Image:Annealing of the two mutated FlhDC strands (sheila).jpg|300px]]<br> | ||
| - | We can se very faint bands in lane 2 and 3, at around 1000bp. The band in lane 3 is a little more visible than lane 2. Because we haven't used primers | + | We can se very faint bands in lane 2 and 3, at around 1000bp, which is the right size. The band in lane 3 is a little more visible than lane 2. Because we haven't used primers in this PCR, the pieces will only have been extended once and we need to ad flhDC fw and rv in order to obtain more product. <br><br> |
--[[User:Sheila|Sheila]] 09:10, 22 July 2010 (UTC) | --[[User:Sheila|Sheila]] 09:10, 22 July 2010 (UTC) | ||
| Line 186: | Line 186: | ||
''Date:'' July 19th <br><br> | ''Date:'' July 19th <br><br> | ||
''Protocol:'' [https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP1.1]<br><br> | ''Protocol:'' [https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP1.1]<br><br> | ||
| - | ''Method:'' The experiment was done in two rounds. First, the annealing product, made by Pernille on | + | ''Method:'' The experiment was done in two rounds. First, the annealing product, made by Pernille on July 19th was amplified. Then we amplified Pernilles amplification simultaneously as we amplified [https://2010.igem.org/Team:SDU-Denmark/labnotes2#Annealing_of_the_two_mutated_strands_of_FlhDC_.28FlhDCmut.29 Sheilas previous annealing].<br> Primers used: FlhDC fw and FlhDC rev <br> Polymerase used: Pfu<br><br> |
''Notes:'' <br><br> | ''Notes:'' <br><br> | ||
''Results:''<br><br> | ''Results:''<br><br> | ||
| Line 198: | Line 198: | ||
''Notes:'' <br><br> | ''Notes:'' <br><br> | ||
''Results:''<br><br> | ''Results:''<br><br> | ||
| - | === Restriction digest of FlhDCmut and pSB1C3 === | + | === Restriction digest of FlhDCmut and pSB1C3 X2 === |
<br> | <br> | ||
''Experiment done by:'' Sheila <br><br> | ''Experiment done by:'' Sheila <br><br> | ||
''Date:'' July 20th <br><br> | ''Date:'' July 20th <br><br> | ||
| - | ''Protocol:'' <br><br> | + | ''Protocol:'' [https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1 RD1.1] <br><br> |
| - | ''Method:'' <br><br> | + | ''Method:'' 'Premixes:'<br> |
| - | '' | + | <html> |
| - | '' | + | <head> |
| - | === Gel extraction of FlhDCmut and pSB1C3 === | + | <meta content="text/html; charset=ISO-8859-1" |
| + | http-equiv="content-type"> | ||
| + | <title></title> | ||
| + | </head> | ||
| + | <body> | ||
| + | <table style="text-align: left; width: 256px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tbody> | ||
| + | <tr> | ||
| + | <td style="width: 116px;"><span | ||
| + | style="font-weight: bold;">FlhDCmut</span></td> | ||
| + | <td style="text-align: center; font-weight: bold; width: 57px;">X1</td> | ||
| + | <td style="text-align: center; font-weight: bold; width: 57px;">X3</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="width: 116px;">H<small><small>2<big><big>O</big></big></small></small></td> | ||
| + | <td style="text-align: right; width: 57px;">12<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | <td style="text-align: right; width: 57px;">3<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">6µL</span><span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US"></span></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="width: 116px;">FD Green Buffer</td> | ||
| + | <td style="text-align: right; width: 57px;">2<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | <td style="text-align: right; width: 57px;">6<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="width: 116px;">Enzyme A: EcoRI</td> | ||
| + | <td style="text-align: right; width: 57px;">1<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | <td style="text-align: right; width: 57px;">3<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="width: 116px;">Enzyme B: S</td> | ||
| + | <td style="text-align: right; width: 57px;">1<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | <td style="text-align: right; width: 57px;">3<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | </tr> | ||
| + | </tbody> | ||
| + | </table> | ||
| + | <br> | ||
| + | <table style="text-align: left; width: 254px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tbody> | ||
| + | <tr> | ||
| + | <td style="font-weight: bold; width: 116px;"><span | ||
| + | style="font-weight: bold;">pSB1C3</span></td> | ||
| + | <td style="font-weight: bold; text-align: center; width: 56px;"><span | ||
| + | style="font-weight: bold;">X1</span></td> | ||
| + | <td style="font-weight: bold; text-align: center; width: 56px;">X3</td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="width: 116px;">H<small><small>2<big><big>O</big></big></small></small></td> | ||
| + | <td style="width: 56px; text-align: right;">12<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | <td style="width: 56px; text-align: right;">36<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="width: 116px;">FD Green Buffer</td> | ||
| + | <td style="width: 56px; text-align: right;">2<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | <td style="width: 56px; text-align: right;">6<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="width: 116px;">Enzyme A: EcoRI</td> | ||
| + | <td style="width: 56px; text-align: right;">1<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | <td style="width: 56px; text-align: right;">3<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="width: 116px;">Enzyme B: X</td> | ||
| + | <td style="width: 56px; text-align: right;">1<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | <td style="width: 56px; text-align: right;">3<span | ||
| + | style="font-size: 11pt; line-height: 115%; font-family: "Calibri","sans-serif";" | ||
| + | lang="EN-US">µL</span></td> | ||
| + | </tr> | ||
| + | </tbody> | ||
| + | </table> | ||
| + | <br> | ||
| + | </body> | ||
| + | </html> | ||
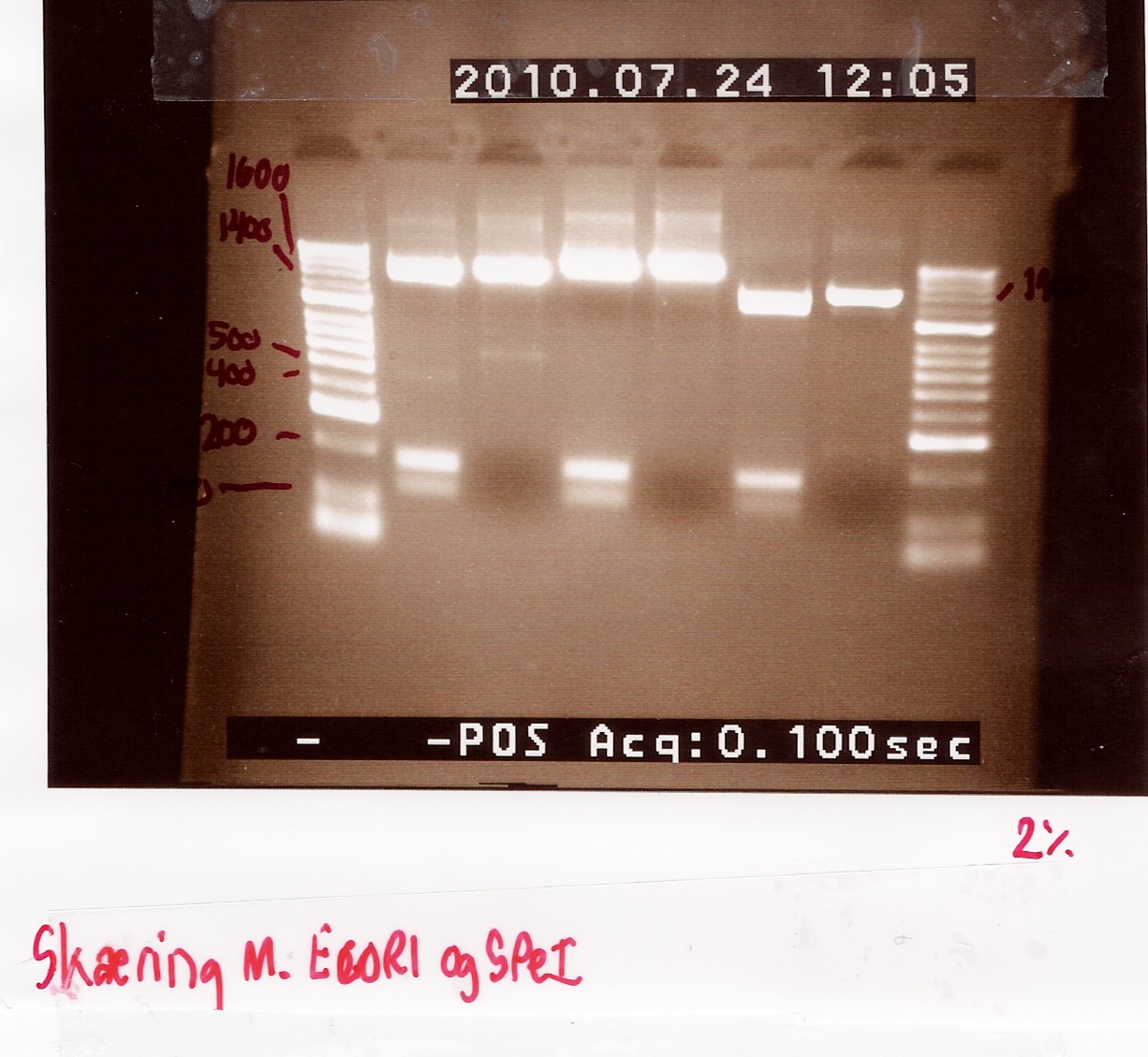
| + | The digested samples were then run on a 1,5% agarose gel for approximately 20 minutes<br><br> | ||
| + | ''Results:'' Because it failed the first time, this experiment was repeated, with the same results (see notes section)<br><br> | ||
| + | '''The first gel'''<br><br> | ||
| + | '''The second gel'''<br><br> | ||
| + | The gels do not show any sign of a successful digest. Because the restiction enzymes only cut in the prefix and suffix of the FlhDCmut operon, it is not surprising that we do not see any bands of approximately 10bp length. However, the digestion should be very clearly visible in the plasmid backbone, as we should be cutting out RFP, which is approximately 1000bp long. The plasmid do not appear to have been digested by the restriction enzymes. 2 bands are visible, but they are both much longer than expected, in fact they appear around the length of the complete plasmid. So this experiment appears to have been unsuccessful.<br><br> | ||
| + | ''Notes:'' First troubleshooting session led to a repeat of the experiment, as we found that human error must have been the reason for the failed results, because it was the first time i performed the experiment. However, when the repeat of the restriction digest yielded the same results, we took a closer look and found that the combination of restriction enzymes was wrong. They were the combination we will use when we begin the assembly... In this case we want to insert the biobrick (FlhDCmut) into the plasmid backbone (pSB1C3, in which case we need to use the same restriction enzymes on both the plasmid and the biobrick. <br><br> | ||
| + | --[[User:Sheila|Sheila]] 22:25, 1 August 2010 (UTC) | ||
| + | |||
| + | === Gel extraction of FlhDCmut and pSB1C3 X2 === | ||
<br> | <br> | ||
''Experiment done by:'' Sheila <br><br> | ''Experiment done by:'' Sheila <br><br> | ||
| - | ''Date:'' July 20th <br><br> | + | ''Date:'' July 20th and July 21st<br><br> |
| - | ''Protocol:'' <br><br> | + | ''Protocol:'' [https://2010.igem.org/Team:SDU-Denmark/protocols#DE1.3 DE1.3]<br><br> |
| - | ''Method:'' <br><br> | + | ''Method:'' The samples were run on a 1,5% agarose gel.<br><br> |
| - | ''Notes:'' <br><br> | + | ''Notes:'' No restriction were seen in the pSB1C3 lane, which lead to the conclusion that the restriction digest failed ([https://2010.igem.org/Team:SDU-Denmark/labnotes2#Restriction_digest_of_FlhDCmut_and_pSB1C3_X2 see above]. The FlhDC was extracted from the gel in order to amplify it with PCR<br><br> |
| - | ''Results:''<br><br> | + | ''Results:'' <br> |
| + | '''The first gel'''<br><br> | ||
| + | '''The second gel'''<br><br> | ||
| + | --[[User:Sheila|Sheila]] 14:42, 2 August 2010 (UTC) | ||
| + | |||
=== Amplification of FlhDCmut === | === Amplification of FlhDCmut === | ||
<br> | <br> | ||
| Line 351: | Line 469: | ||
''Results:''<br> | ''Results:''<br> | ||
Gel electrophoresis:<br> | Gel electrophoresis:<br> | ||
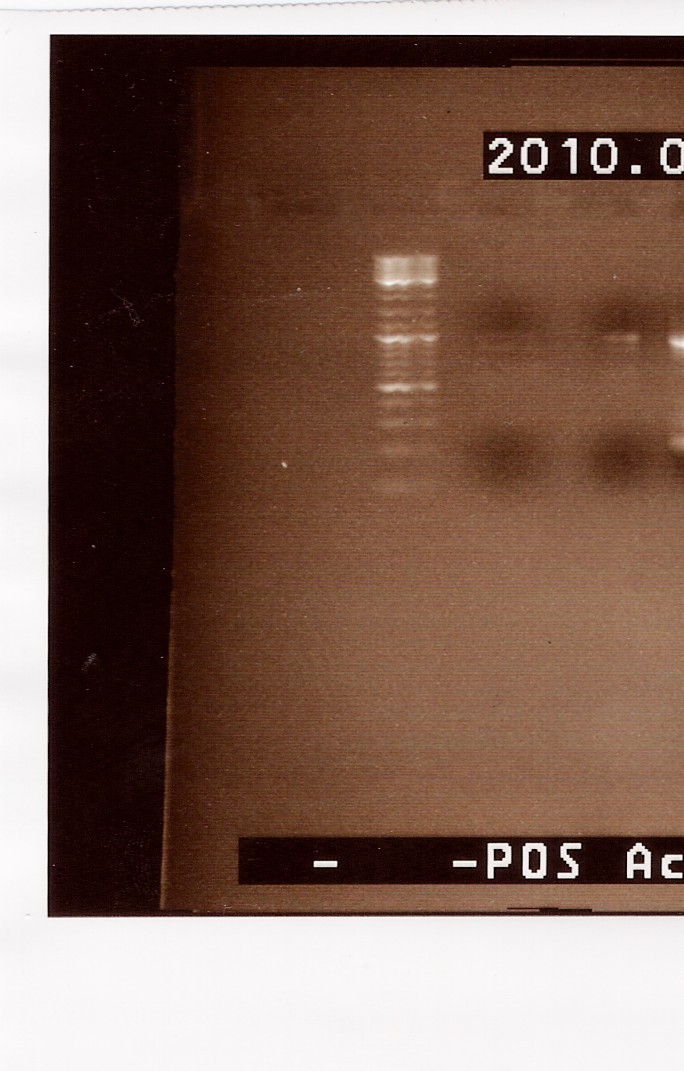
| - | [[Image:Team-SDU-Denmark-FlhD,C digestion with SpeI and EcoRI.jpg | | + | [[Image:Team-SDU-Denmark-FlhD,C digestion with SpeI and EcoRI.jpg |300px]]<br><br> |
''Analysis:''<br> | ''Analysis:''<br> | ||
In lane 2 containing pSB1C3 2 bands are detected indicating a succesful digestion of the plasmid (the band at 1000 bp corresponds to J04450). A succesful digestion of the flhD/C cannot be concluded from the gel. However both bands was excised and extracted from gel ([https://2010.igem.org/Team:SDU-Denmark/labnotes2#Gel_extraction_of_digested_flhD.2FC_and_pSB1C3 Gel extraction]) <br> | In lane 2 containing pSB1C3 2 bands are detected indicating a succesful digestion of the plasmid (the band at 1000 bp corresponds to J04450). A succesful digestion of the flhD/C cannot be concluded from the gel. However both bands was excised and extracted from gel ([https://2010.igem.org/Team:SDU-Denmark/labnotes2#Gel_extraction_of_digested_flhD.2FC_and_pSB1C3 Gel extraction]) <br> | ||
| Line 475: | Line 593: | ||
Ligation mixtures were not run on gel but were directly used for [https://2010.igem.org/Team:SDU-Denmark/labnotes2#Transformation_of_flhD.2FC_in_pSB1C3_and_test_plasmid_in_Top_10_E.Coli transformation]<br> | Ligation mixtures were not run on gel but were directly used for [https://2010.igem.org/Team:SDU-Denmark/labnotes2#Transformation_of_flhD.2FC_in_pSB1C3_and_test_plasmid_in_Top_10_E.Coli transformation]<br> | ||
surplus ligation mixture was stored at -20 degrees as L1, L2 and L3.<br> | surplus ligation mixture was stored at -20 degrees as L1, L2 and L3.<br> | ||
| - | --[[User:Tipi|Tipi]] 18:15, 19 July 2010 (UTC) | + | --[[User:Tipi|Tipi]] 18:15, 19 July 2010 (UTC)<br><br> |
| + | |||
| + | === Coloni PCR of flhD/C (native coding sequence) in pSB1C3 === | ||
| + | Start date: 21/07 End date: 21/07<br> | ||
| + | ''Methods:'' Coloni PCR and gel electrophoresis <br><br> | ||
| + | ''Protocol:''[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.2 CP1.2] <br><br> | ||
| + | ''Experiment done by:'' Maria and LC<br><br> | ||
| + | ''Notes:''<br> | ||
| + | Coloni PCR was made on flhD/C in pSB1C3 from [https://2010.igem.org/Team:SDU-Denmark/labnotes2#Transformation_of_flhD.2FC_in_pSB1C3_and_test_plasmid_in_Top_10_E.Coli Transformation].<br> | ||
| + | 10 colonies from plates plated with cells transformed with L2 and L3 ligation mixtures (see [https://2010.igem.org/Team:SDU-Denmark/labnotes2#Ligation_of_flhD.2FC_.28native_coding_sequence.29_and_pSB1C3 Ligation]).<br> | ||
| + | Coloni 1-5 is taken from L2 plates | ||
| + | coloni 6-10 is taken from L3 plates <br> | ||
| + | PCR was made with only half the amount of premix.<br> | ||
| + | Premix for 12 PCR reactions: <br> | ||
| + | <table style="text-align: left; width: 100px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">10xtaq buffer<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">30uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">MgCl2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">12uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">VF2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">12uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">VR<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">12uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">dNTP<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">6uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">H2O<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">42uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Total vol.<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">114uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> <br><br> | ||
| + | 0.5uL Taq polymerase from ampliqon was used.<br> | ||
| + | Taq PCR program:<br> | ||
| + | <table style="text-align: left; height: 260px; width: 225px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">1:Start<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">94C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">2 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">2: Denaturing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">94C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">1 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">3: Annealing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">55C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">1 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">4: Elongation<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">72C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">1 min 30 s<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">5:<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">GO TO<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">rep. 29x<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">6: End <br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">72C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">3 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">7: Hold<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">4C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;"><br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> <br><br> | ||
| + | PCR product was loaded onto a 2% agarose gel. Gene ruler DNA ladder mix (red) was used as marker.<br><br> | ||
| + | VF2 - coding sequence (in pSB1C3) : 140bp <br> | ||
| + | Coding sequence - VR (in pSB1C3) : 176bp <br> | ||
| + | flhD/C : 932bp <br> | ||
| + | VF2 - VR for pSB1C3 w. flhD/C :1248bp <br><br> | ||
| + | VF2 - VR for pSB1C3 without insertion : 316bp <br><br> | ||
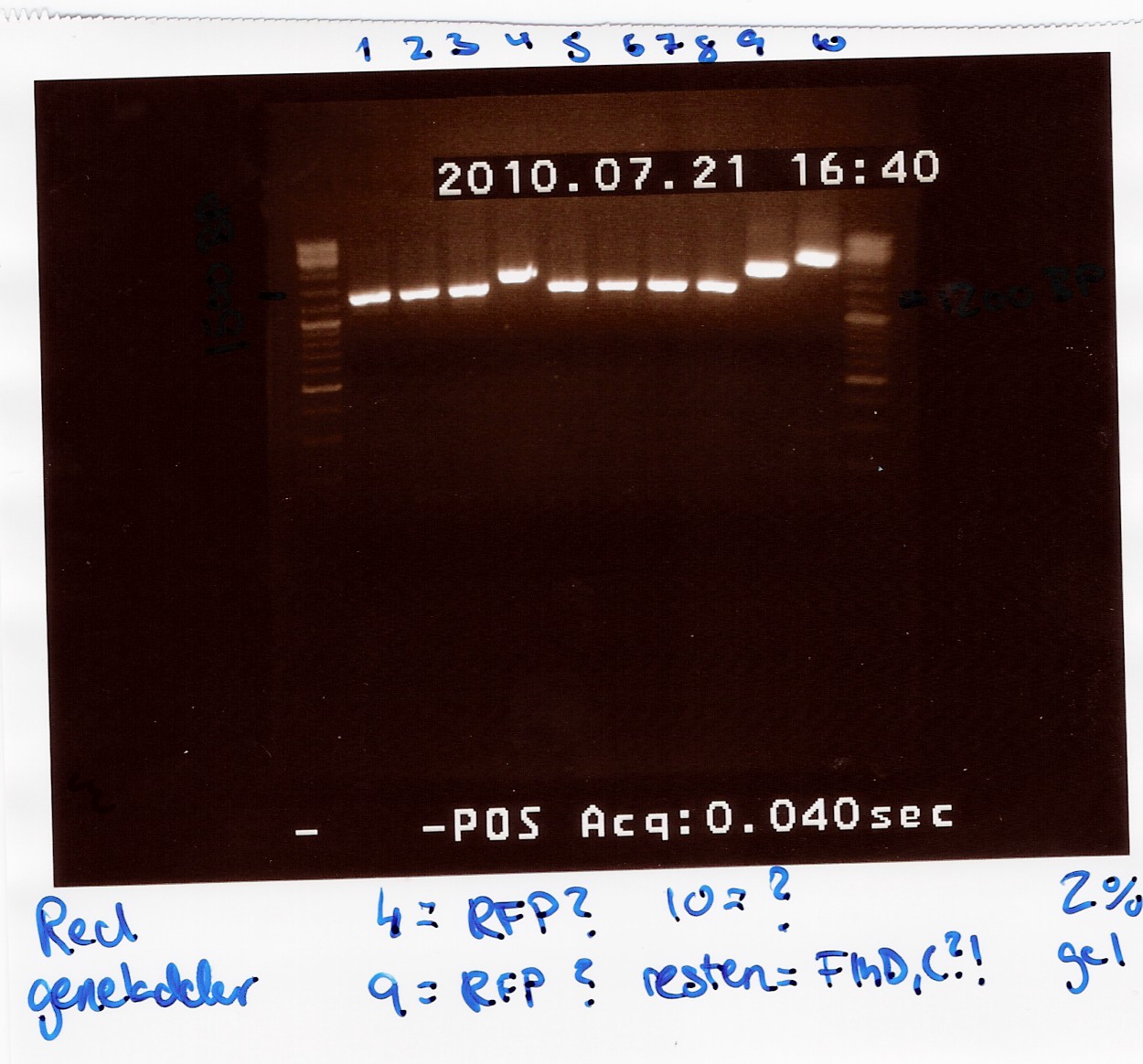
| + | ''Results:''<br> | ||
| + | Gel electrophoresis: <br> | ||
| + | [[Image:Team-SDU-Denmark-coloni PCR flhDC ligation.jpg|300px]]<br><br> | ||
| + | |||
| + | ''Analysis:''<br> | ||
| + | All lanes except 4, 9 and 10 showed a band at app. 1200bp. which might indicate plasmids where flhD/C has been inserted. | ||
| + | Lane 4 and 9 has a band at app. 1500bp which could indicate undigested pSB1C3 (VF2 - VR for pSB1C3 w. J04450 is 1385bp).<br> | ||
| + | Streak plates of coloni 1, 2, 3, 5, 6, 7, 8 and 10 showed red colonies, indicating that these contained undigested pSB1C3.<br> Streak plates of coloni 4 and 9 had only white colonies, suggesting an insertion has occured.<br> To verify if flhD/C has been inserted coloni PCR using VF2 and flhD/C rev. as primers was carried out for colonies from streak plates of coloni 4 and 9 and of all white colonies from the transformation plates. (see coloni PCR)<br> | ||
| + | --[[User:Tipi|Tipi]] 11:46, 24 July 2010 (UTC) <br><br> | ||
| + | |||
| + | === Coloni PCR of flhD/C (native coding sequence) in pSB1C3 === | ||
| + | Start date: 22/07 End date: 22/07<br> | ||
| + | ''Methods:'' Coloni PCR and gel electrophoresis <br><br> | ||
| + | ''Protocol:''[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.3 CP1.3] <br><br> | ||
| + | ''Experiment done by:'' Maria<br><br> | ||
| + | ''Notes:''<br> | ||
| + | Coloni PCR was made on flhD/C in pSB1C3 from [https://2010.igem.org/Team:SDU-Denmark/labnotes2#Transformation_of_flhD.2FC_in_pSB1C3_and_test_plasmid_in_Top_10_E.Coli Transformation].<br> | ||
| + | All remaining white colonies from plates plated with cells transformed with L2 and L3 ligation mixtures (see [https://2010.igem.org/Team:SDU-Denmark/labnotes2#Ligation_of_flhD.2FC_.28native_coding_sequence.29_and_pSB1C3 Ligation]) was selected.<br> | ||
| + | coloni 1-6 is taken from L2.3 plates<br> | ||
| + | coloni 7-15 is taken from L3.2 plates <br> | ||
| + | coloni 16-27 is taken from L3.3 plates<br> | ||
| + | coloni 28 is taken from streak plate of PCR no. 4 | ||
| + | coloni 29 is taken from streak plate of PCR no. 9 (see [https://2010.igem.org/Team:SDU-Denmark/labnotes2#Coloni_PCR_of_flhD.2FC_in_pSB1C3_2 Coloni PCR])<br> | ||
| + | Premix for 31 PCR reactions: <br> | ||
| + | <table style="text-align: left; width: 100px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">10xtaq buffer<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">77.5uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">MgCl2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">31uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">VF2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">31uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">flhD/C rev.<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">31uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">dNTP<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">15.5uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">H2O<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">108.5uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Total vol.<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">294.5uL<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> <br><br> | ||
| + | 0.5uL Taq polymerase from fermentas was used.<br> | ||
| + | Taq PCR program:<br> | ||
| + | <table style="text-align: left; height: 260px; width: 225px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">1:Start<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">94C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">2 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">2: Denaturing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">94C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">1 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">3: Annealing<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">55C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">1 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">4: Elongation<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">72C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">1 min 30 s<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">5:<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">GO TO<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">rep. 29x<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">6: End <br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">72C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;">3 min<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 102px;">7: Hold<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 52px;">4C<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 45px;"><br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> <br><br> | ||
| + | PCR product was loaded onto a 2% agarose gel. Gene ruler DNA ladder mix (red) was used as marker.<br><br> | ||
| + | |||
| + | ''Results:''<br> | ||
| + | Gel electrophoresis: <br> | ||
| + | [[Image:Team-SDU-Denmark-Coloni PCR 220710.jpg|300 px]]<br><br> | ||
| + | |||
| + | ''Analysis:''<br> | ||
| + | None of the samples contained amplified DNA.<br> | ||
| + | This indicates either that the flhD/C gene has not been inserted to the pSB1C3 backbone or an insertion has been made but the flhD/C primer has not been able to anneal to the template.<br> | ||
| + | To verify that a ligation has occurred, PCR product 4 and 9 from [https://2010.igem.org/Team:SDU-Denmark/labnotes2#Coloni_PCR_of_flhD.2FC_in_pSB1C3 Coloni PCR] was digested with EcoRI and SpeI.<br> | ||
| + | --[[User:Tipi|Tipi]] 13:53, 24 July 2010 (UTC)<br><br> | ||
| + | |||
| + | === Digestion of flhD/C PCR product with EcoRI and SpeI === | ||
| + | Start date: 24/07 End date: 24/07<br> | ||
| + | ''Methods:'' Restriction digest and gel electrophoresis <br><br> | ||
| + | ''Protocol:''[https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1 RD1.1] <br><br> | ||
| + | ''Experiment done by:'' Maria<br><br> | ||
| + | ''Notes:''<br> | ||
| + | To verify if a ligation has occurred Taq PCR product of coloni 4 and 9 (see [https://2010.igem.org/Team:SDU-Denmark/labnotes2#Coloni_PCR_of_flhD.2FC_in_pSB1C3 Coloni PCR]) was digested with the same restiction enzymes used for the digestion of flhD/C DNA and pSB1C3 prior to ligation.<br> If an ligation has occurred, then the restriction sites of EcoRI and SpeI should be intact, and two fragments of 122bp and 170bp should be observed on the gel, indicating VF2-E site and S site-VR respectively.<br> | ||
| + | RFP (PCR poduct of coloni no.8) was used as controle.<br> | ||
| + | 2.5uL of undigested PCR product of coloni 4, 8 and 9 were loaded onto the gel as well.<br> | ||
| + | Samples were loaded onto a 2% agarose gel. Hyper Ladder II was used as marker.<br> | ||
| + | Loading scheme:<br> | ||
| + | <table style="text-align: left; width: 100%;" border="1" cellpadding="2" | ||
| + | cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Lane<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">3<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">4<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">5<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">6<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;"><br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">digested no. 4<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">undigested no. 4<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">digested no. 9<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">undigested no. 9<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">digested no. 8<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">undigested no. 8<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table><br><br> | ||
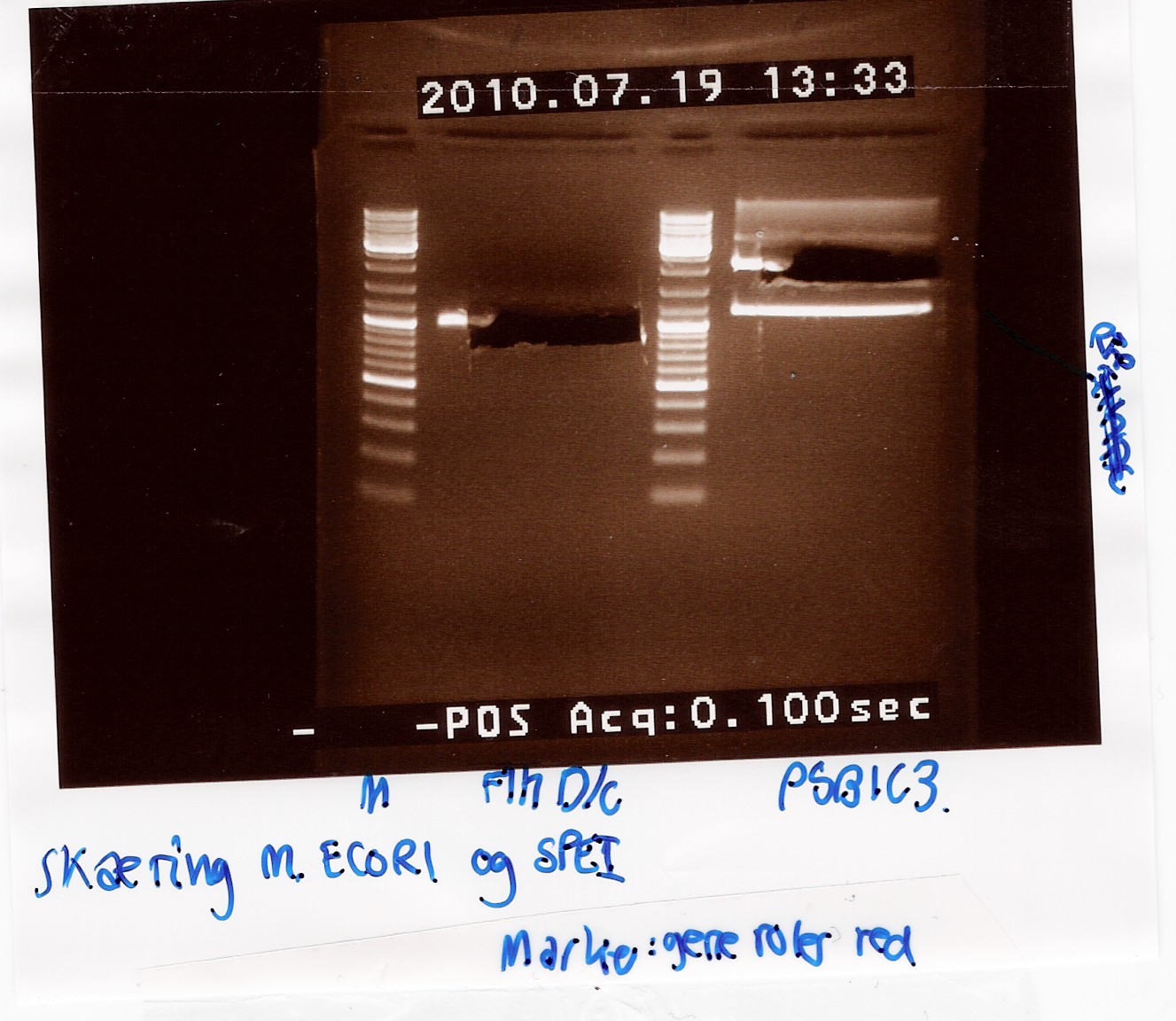
| + | ''Results:''<br> | ||
| + | Gel electrophoresis:<br> | ||
| + | [[Image:Team-SDU-Denmark-Digestion of coloni PCR product-1.jpg|300px]]<br><br> | ||
| + | ''Analysis:''<br> | ||
| + | The 2 bands at app. 100bp and 200bp respectively indicates that the E and S restiction sites have been preserved, suggesting that a ligation has occured.<br> | ||
| + | An ON culture of coloni 4 and 9 is made to use for mini-prep.<br> | ||
| + | --[[User:Tipi|Tipi]] 14:37, 24 July 2010 (UTC)<br><br> | ||
| + | |||
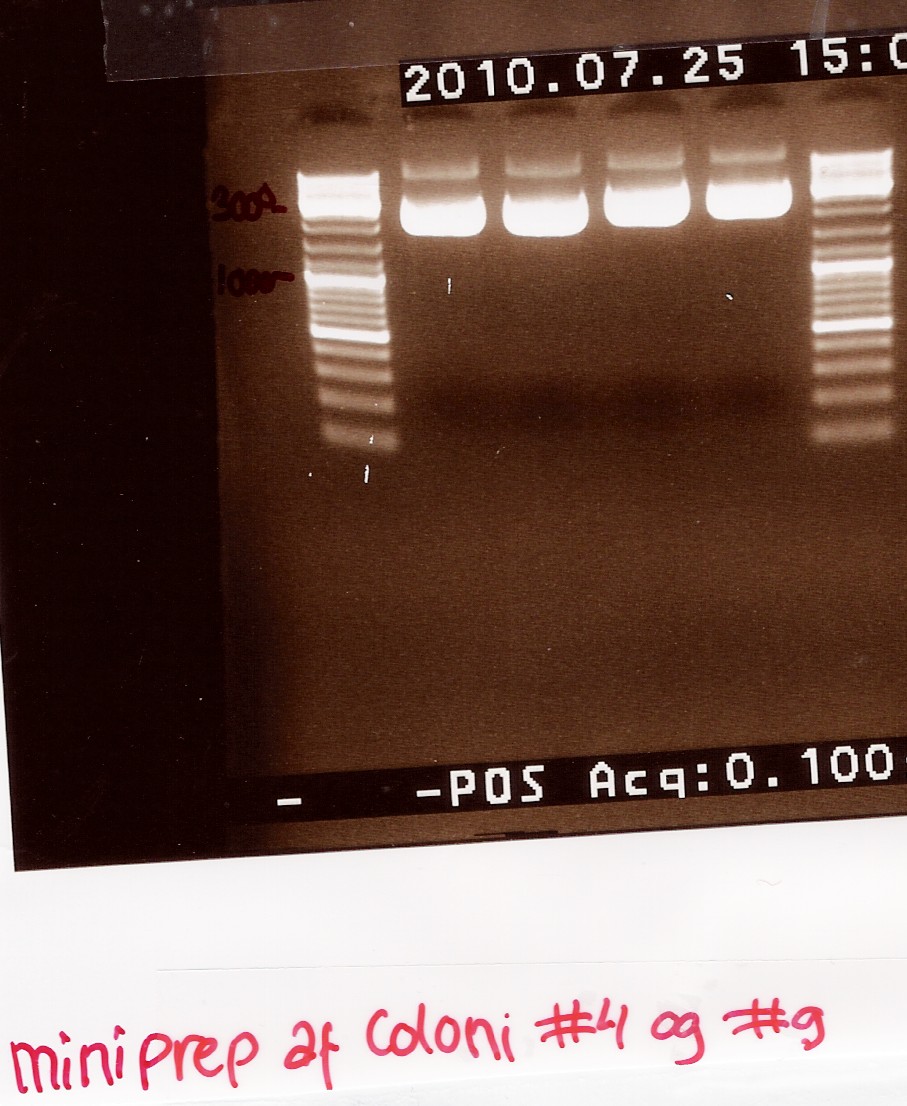
| + | === Miniprep of ligation coloni #4 and #9 === | ||
| + | Start date: 25/07 End date: 25/07<br> | ||
| + | ''Methods:'' Mini prep and nanodrop <br><br> | ||
| + | ''Protocol:''[https://2010.igem.org/Team:SDU-Denmark/protocols#MP1.1 MP1.1] <br><br> | ||
| + | ''Experiment done by:'' Maria<br><br> | ||
| + | ''Notes:''<br> | ||
| + | The samples are loaded onto a 1.5% agarose gel. Gene ruler DNA ladder mix (red) is used as marker.<br> | ||
| + | Loading sheme:<br> | ||
| + | <table style="text-align: left; width: 300px;" border="1" cellpadding="2" | ||
| + | cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Lanes<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">3<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">4<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;"><br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;"># 4.1<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;"># 4.2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;"># 9.1<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;"># 9.2<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table><br><br> | ||
| + | |||
| + | ''Results:''<br> | ||
| + | Gel electrophoresis:<br> | ||
| + | [[Image:Team-SDU-Denmark-Miniprep ligering 4 og 9.jpg|300px]]<br><br> | ||
| + | Nanodrop:<br> | ||
| + | <table style="text-align: left; width: 100px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Sample ID<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">ng/uL<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">260/280<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">260/230<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">miniprep coloni #4.1<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">115.68<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1.91<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2.17<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">miniprep coloni #4.2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">115.74<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1.91<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2.13<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">miniprep coloni #9.1<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">131.32<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1.92<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2.23<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">miniprep coloni #9.2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">119<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1.93<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2.16<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">miniprep coloni #4 pooled<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">116.51<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1.92<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2.15<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">miniprep coloni #9 pooled<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">123.94<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1.93<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2.17<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | ''Analysis:'' | ||
| + | We have purified the plasmids. The concentrations however are too low for sequencing, so the samples are dried down to reach a conc. of 200ng/uL <br> | ||
| + | --[[User:Tipi|Tipi]] 08:44, 28 July 2010 (UTC)<br><br> | ||
| + | |||
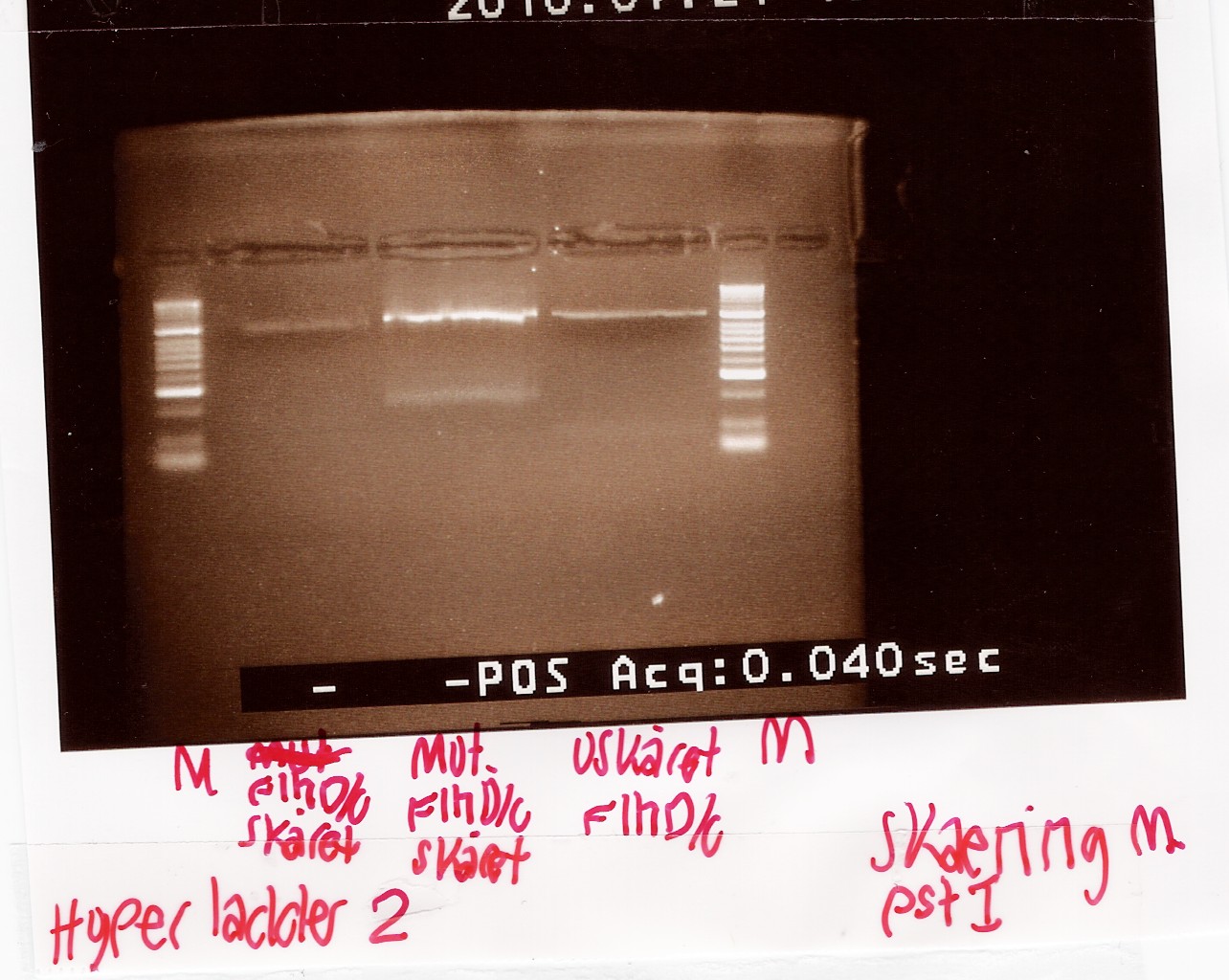
| + | === Digestion of flhD/C mut with PstI === | ||
| + | ====Experiment no.1==== | ||
| + | Start date: 21/07 End date: 21/07<br> | ||
| + | ''Methods:'' Restriction digest and gel electrophoresis <br><br> | ||
| + | ''Protocol:''[https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1 RD1.1] <br><br> | ||
| + | ''Experiment done by:'' Maria<br><br> | ||
| + | ''Notes:''<br> | ||
| + | To verify if the silent mutation has been made PCR product of flhD/C mut (see [[https://2010.igem.org/Team:SDU-Denmark/labnotes2#Amplification_of_FlhDCmut flhD/C mut PCR]] was digested with restiction enzyme PstI.<br> | ||
| + | If the mutation has been made the flhD/C fragment should not be digested with PstI.<br> | ||
| + | PCR product of flhD/C (native coding sequence) was used as controle.<br> | ||
| + | Undigested PCR product of flhD/C (native coding sequence) was loaded onto the gel as well.<br>Samples were loaded onto a 2% agarose gel. Hyper Ladder II was used as marker.<br> | ||
| + | Loading scheme:<br> | ||
| + | <table style="text-align: left; width: 100%;" border="1" cellpadding="2" | ||
| + | cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Lane<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">3<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;"><br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">Digested flhD/C (native coding sequence) <br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">Digested flhD/C mut<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">Undigested flhD/C (native coding sequence)<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table><br><br> | ||
| + | ''Results:''<br> | ||
| + | Gel electrophoresis:<br> | ||
| + | [[Image:Team-SDU-Denmark-Digestion test flhD,C.jpg|300px]]<br><br> | ||
| + | ''Analysis:''<br> | ||
| + | The intensity of the bands are too low to conclude anything. The experiment was remade using a gel with smaller wells.<br> | ||
| + | --[[User:Tipi|Tipi]] 14:37, 24 July 2010 (UTC)<br><br> | ||
| + | |||
| + | ====experiment no.2==== | ||
| + | Start date: 22/07 End date: 22/07<br> | ||
| + | ''Methods:'' Restriction digest and gel electrophoresis <br><br> | ||
| + | ''Protocol:''[https://2010.igem.org/Team:SDU-Denmark/protocols#RD1.1 RD1.1] <br><br> | ||
| + | ''Experiment done by:'' Maria<br><br> | ||
| + | ''Notes:''<br> | ||
| + | Taq PCR product of flhD/C (native coding sequence) (no.5 green) was used as controle.<br> | ||
| + | Undigested PCR product of flhD/C (native coding sequence) was loaded onto the gel as well.<br>Samples were loaded onto a 2% agarose gel. Hyper Ladder II was used as marker.<br> | ||
| + | Loading scheme:<br> | ||
| + | <table style="text-align: left; width: 100%;" border="1" cellpadding="2" | ||
| + | cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Lane<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">3<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;"><br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">Digested flhD/C (native coding sequence) <br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">Digested flhD/C mut<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">Undigested flhD/C (native coding sequence)<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table><br><br> | ||
| + | ''Results:''<br> | ||
| + | Gel electrophoresis:<br> | ||
| + | [[Image:Team-SDU-Denmark-Digestion test flhD,C-1.jpg|300px]]<br><br> | ||
| + | ''Analysis:''<br> | ||
| + | A small fragment is be observed for both the flhD/C mut and flhD/C (native coding sequence), so it cannot be fully concluded that the mutation has been made. Another digestion reaction must be carried out.<br> | ||
| + | --[[User:Tipi|Tipi]] 15:46, 24 July 2010 (UTC) | ||
===Purification of pSB1A2 containing J13002=== | ===Purification of pSB1A2 containing J13002=== | ||
| Line 481: | Line 1,117: | ||
''Experiment done by:'' Pernille <br><br> | ''Experiment done by:'' Pernille <br><br> | ||
''Date:'' the 19 of July <br><br> | ''Date:'' the 19 of July <br><br> | ||
| - | ''Protocol:'' [https://2010.igem.org/Team:SDU-Denmark/protocols#Plasmid_miniprep_kit_.28Fermentas.29 MP1.1]<br><br> | + | ''Protocol:'' [https://2010.igem.org/Team:SDU-Denmark/protocols#Plasmid_miniprep_kit_.28Fermentas.29 MP1.1] and [https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP1.1]<br><br> |
| - | ''Method:'' Miniprep, Nano drop | + | ''Method:'' Miniprep, Nano drop, gel electrophoresis and PCR <br><br> |
''Notes:'' The pSB1A2 plasmid are a high-copy-plasmid and therefore 5mL ON culture are used for purification of the plasmid. for the PCR the following PRC program are used: | ''Notes:'' The pSB1A2 plasmid are a high-copy-plasmid and therefore 5mL ON culture are used for purification of the plasmid. for the PCR the following PRC program are used: | ||
1: 94°C 3min | 1: 94°C 3min | ||
| Line 492: | Line 1,128: | ||
7: hold 4°C | 7: hold 4°C | ||
<br><br> | <br><br> | ||
| - | ''Results:'' | + | ''Results:''After the purification the concentration of nucleic acid concentration was measured on the Nano Drop. There was made four purification tubes. Only tube 1 and 3 was used for further experiments because the other contained a very low concentration of nucleic acid. The concentration in tube 1 and 3 was 25,72ng/uL and 25,76ng/uL. |
| - | The purified DNA was loaded in a 1,5% agarose gel together with a 10.000bp long ladder (red). | + | The purified DNA was loaded in a 1,5% agarose gel together with a 10.000bp long ladder (red).<br> |
| + | [[Image:Team-sdu-denmark-Oprenset psb1A2 medJ13002-1.jpg |300px ]]<br> | ||
PCR was run on the DNA using the VR and VF2 primers wich ensures the only the brick part are amplificated. The pfu polymerase was used but unfortunately i did not get any results since i did not add any MgSO4 to the premix.<br><br> | PCR was run on the DNA using the VR and VF2 primers wich ensures the only the brick part are amplificated. The pfu polymerase was used but unfortunately i did not get any results since i did not add any MgSO4 to the premix.<br><br> | ||
--[[User:Pernm07|Pernm07]] 08:48, 22 July 2010 (UTC) | --[[User:Pernm07|Pernm07]] 08:48, 22 July 2010 (UTC) | ||
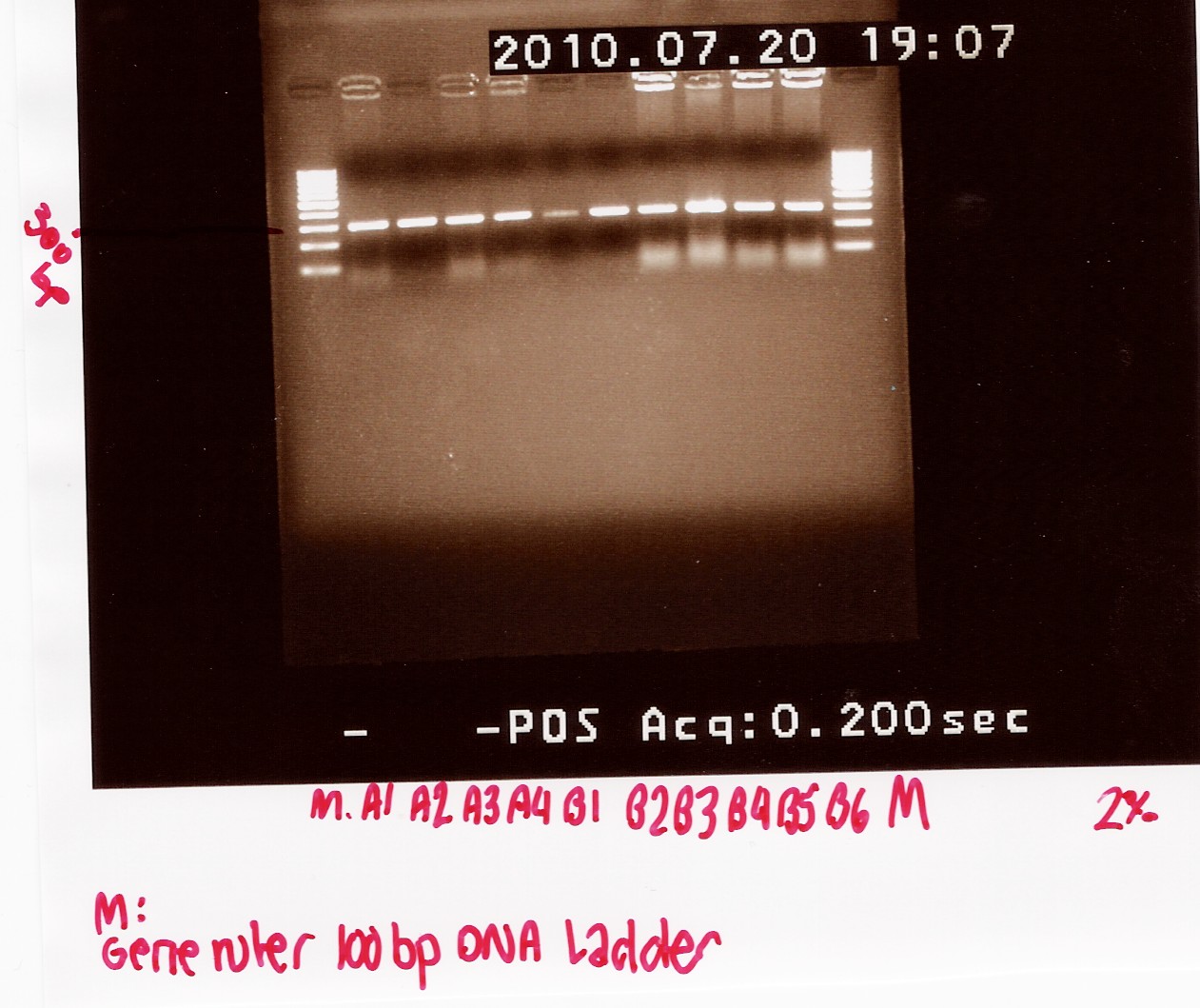
| - | ===Amplification of pSB1A2 w. J13002, pSB1A2 w. B0015 and pSB1A2 w. B0034=== | + | ===Amplification of pSB1A2 w. [http://partsregistry.org/Part:BBa_J13002 J13002], pSB1A2 w. [http://partsregistry.org/Part:BBa_B0015 B0015] and pSB1A2 w. [http://partsregistry.org/Part:BBa_B0034 B0034]=== |
<br> | <br> | ||
''Experiment done by:'' Pernille <br><br> | ''Experiment done by:'' Pernille <br><br> | ||
| Line 504: | Line 1,141: | ||
''Method:'' PCR and DNA extraction <br><br> | ''Method:'' PCR and DNA extraction <br><br> | ||
''Notes:'' The purified DNA was amiplificated with PRC using the following program: | ''Notes:'' The purified DNA was amiplificated with PRC using the following program: | ||
| - | + | <br><br> | |
| - | 2: | + | <html><table style="text-align: left; width: 334px;" border="1" |
| - | 3: | + | cellpadding="2" cellspacing="2"> |
| - | + | <tbody> | |
| - | + | <tr> | |
| - | + | <td style="vertical-align: top;">Temperature (Celcius)<br> | |
| - | + | </td> | |
| - | + | <td style="vertical-align: top; width: 99px;">Time (min)<br> | |
| - | + | </td> | |
| - | + | <td style="vertical-align: top; width: 56px;">Description<br> | |
| - | + | </td> | |
| - | + | </tr> | |
| + | <tr> | ||
| + | <td style="vertical-align: top;">94<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 99px;">3<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 56px;">Initialization</td> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">94<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 99px;">2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 56px;">Denaturation</td> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">55<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 99px;">½<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 56px;">Annealing</td> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">72<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 99px;">1<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 56px;">Elongation<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">-<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 99px;">x 29<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 56px;">GOTO step 2<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">72<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 99px;">5<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 56px;">Final Elongation</td> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">4<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 99px;"> <br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 56px;">Hold</td> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </tbody> | ||
| + | </table></html> | ||
| + | <br><br> | ||
| + | the experiment for each biobrick was do in duplicats.<br><br> | ||
| + | ''Results:''After amplification the nucleic acid concentration was measured on the Nano Drop. The concentrations was measued to: | ||
| + | <br> | ||
tube 1: pSB1A2 w. J13002 was 825.2ng/uL | tube 1: pSB1A2 w. J13002 was 825.2ng/uL | ||
| - | tube 2: pSB1A2 w. B0015 was 547.76ng/ | + | tube 2: pSB1A2 w. B0015 was 547.76ng/uL |
tube 3: pSB1A2 w. B0034 was 777.84ng/uL | tube 3: pSB1A2 w. B0034 was 777.84ng/uL | ||
| - | + | <br> | |
| - | The PCR product was loaded on a 2% agarose gel together with a 1000 bp long marker. | + | The PCR product was loaded on a 2% agarose gel together with a 1000 bp long marker.<br> |
| - | + | [[Image:Team-SDU-Denamrk-Promotor+rbs;rbs;db-terminator.jpg|300px]]<br><br> | |
| - | + | During the DNA extraction 50uL DNA anf 7uL loading buffer was loaded in a lane. The samples was loaded from left to right in continuous numbers. <br> | |
| - | During the DNA extraction 50uL DNA anf 7uL loading buffer was loaded in a lane. The samples was loaded from left to right in continuous numbers. | + | [[Image:team-sdu-denmark-Gelextration;promotor+rbs,rbs,dbterminator.jpg|300px]]<br><br> |
| - | + | The concentration of the extracted DNA from tube 1,2 and was found to 13.60ng/uL, 16,58ng/uL and 12,6313.60ng/uL respectively. while we have to use the dobbelt terminator and the constitutive active promotor in a lot of experiment the next step is to do another round of PRC and DNA extraction on thise biobricks.<br><br> | |
| - | The concentration of the extracted DNA from tube 1,2 and was found to 13.60ng/uL, 16,58ng/uL and 12,6313.60ng/uL respectively. while we have to use the dobbelt terminator and the constitutive active promotor in a lot of experiment the next step is to do another round of PRC and DNA extraction on thise biobricks. | + | |
--[[User:Pernm07|Pernm07]] 10:06, 22 July 2010 (UTC) | --[[User:Pernm07|Pernm07]] 10:06, 22 July 2010 (UTC) | ||
| + | |||
| + | ===Amplification and purification of pSB1A2 w. J13002=== | ||
| + | <br> | ||
| + | ''Experiment done by:'' Pernille <br><br> | ||
| + | ''Date:'' The 22 of July <br><br> | ||
| + | ''Protocol:'' [https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP1.1]and [https://2010.igem.org/Team:SDU-Denmark/protocols#DNA_extraction_from_gel_.28fermentas.29 DNA extraction] <br><br> | ||
| + | ''Method:'' PCR and DNA extraction <br><br> | ||
| + | ''Notes:'' The DNA purified in the last experiment was amplified with PRC using VR og VF2 primers and pfu. the normal pfu program was used for running PCR. The PRC product was loaded on a 2% agarose gel. the brick J13002 (promotor+RBS) are 312bp long wich correspond to the visible band on the gel.<br><br> | ||
| + | [[Image:Team-sdu-denmark-Gelextraktionpromotor+rbs.jpg|300px]]<br><br> | ||
| + | The DNA was extracted and afterwards the DNA concentration was measured on Nanodrop. the concentration was found to be 13,02ng/uL. The DNA was saved and it was cut with restriction enzymes. | ||
== Group: Photosensor == | == Group: Photosensor == | ||
| + | === Miniprep of [http://partsregistry.org/Part:BBa_K081005 K081005] in pSB1A2 and [http://partsregistry.org/Part:BBa_R0011 R0011] in pSB1A2 from transformation 20/7 === | ||
| + | Start date: July 22nd<br> | ||
| + | ''Methods:'' Miniprep, gel electrophoresis and nano drop <br><br> | ||
| + | ''Protocol:''[https://2010.igem.org/Team:SDU-Denmark/protocols#MP1.2 MP1.2] <br><br> | ||
| + | ''Experiment done by:'' LC<br><br> | ||
| + | ''Notes:'' / <br><br> | ||
| + | ''Results:''<br> | ||
| + | Gel electrophoresis: <br> | ||
| + | [[Image:Team-SDU-Denmark-miniprep2207.jpg|300px]]<br> | ||
| + | Nanodrop: <br> | ||
| + | The concentration was around 23 ng/ul for all samples. Not overwhelming, but usable.<br><br> | ||
| + | |||
| + | ''Analysis:''<br> | ||
| + | Sample 1-4 of K081005 were pooled and frozen and sample 1-4 of R0011 were too.<br> | ||
<br> | <br> | ||
| + | |||
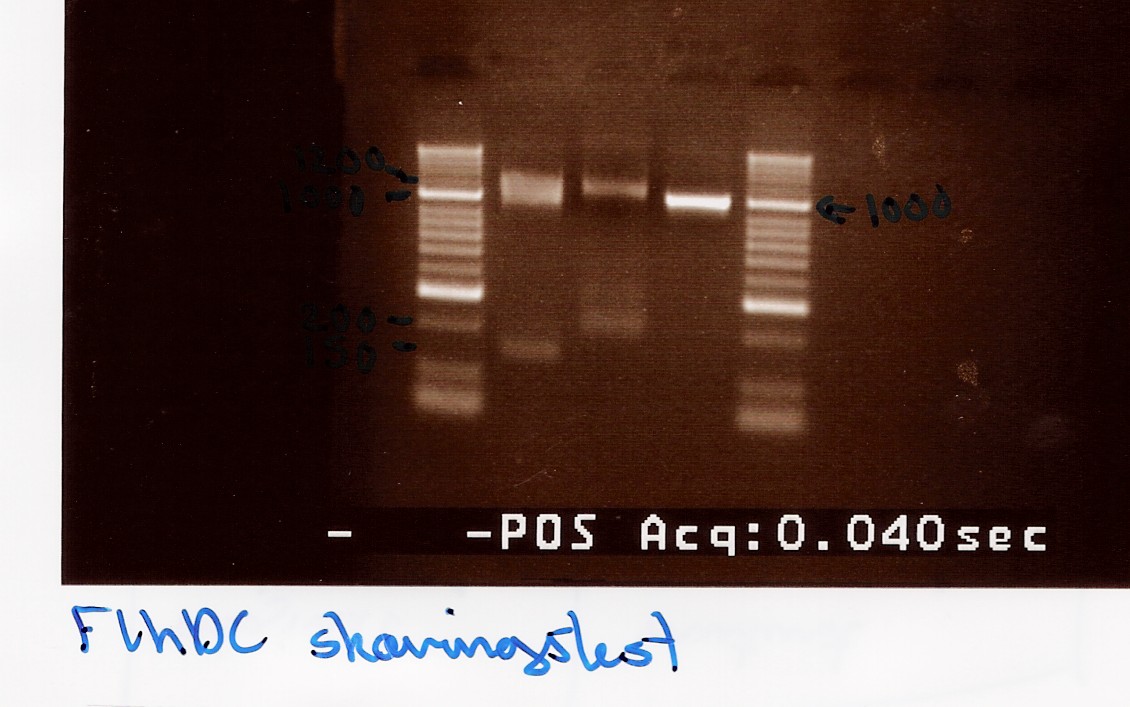
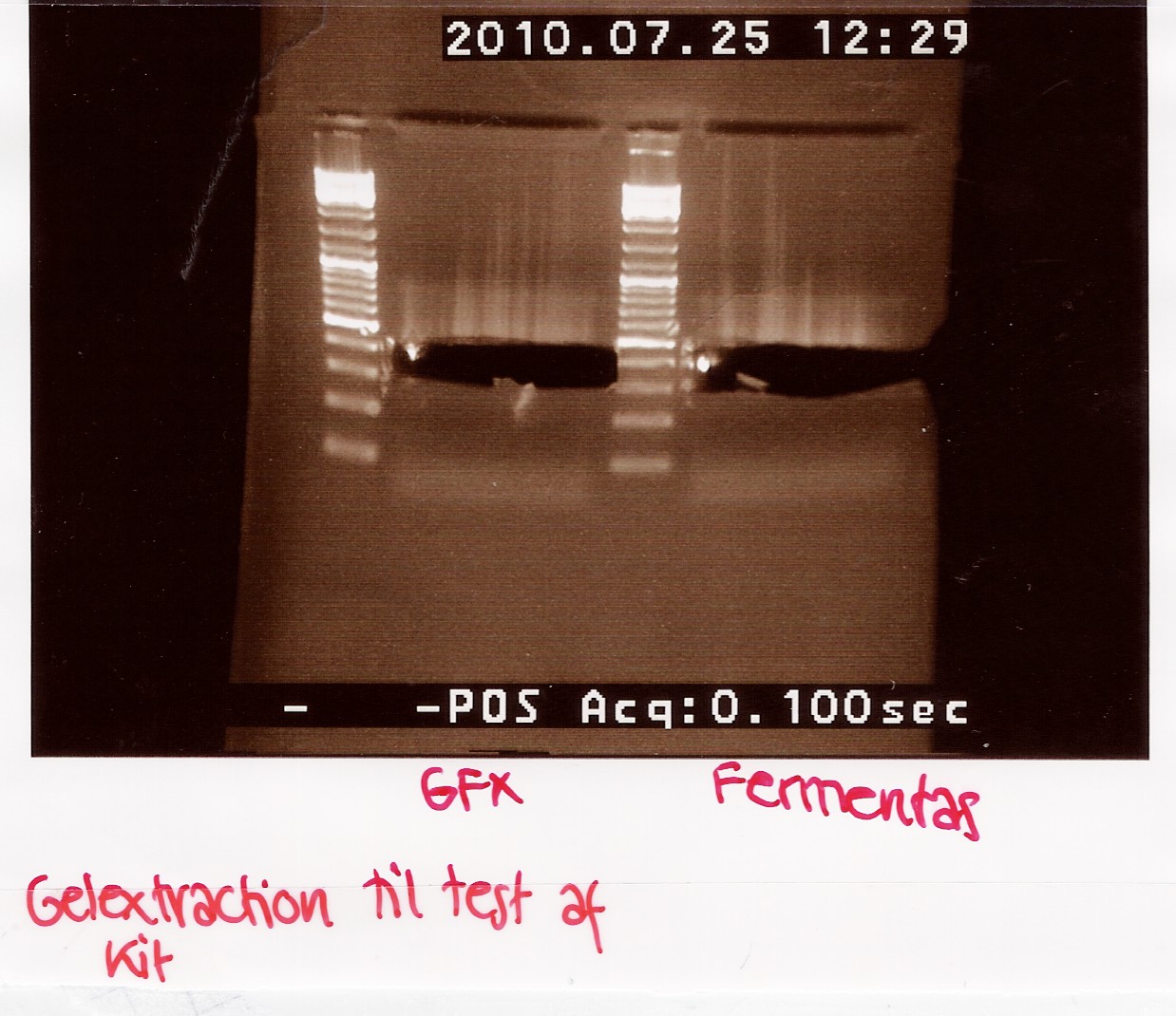
| + | === Gel extraction test (GFX vs. Fermentas) === | ||
| + | Start date: July 25th <br> | ||
| + | ''Methods:'' Gel electrophoresis, gel extraction and nano drop <br><br> | ||
| + | ''Protocol:''[https://2010.igem.org/Team:SDU-Denmark/protocols#DE1.2 DE1.2][https://2010.igem.org/Team:SDU-Denmark/protocols#DE1.3 DE1.3] <br><br> | ||
| + | ''Experiment done by:'' Maria<br><br> | ||
| + | ''Notes:'' <br><br> | ||
| + | FlhD/C taq PCR product (5 green) is used in the test.<br> | ||
| + | PCR product is diluted in H2O to reach a sample concentration below 100ng/uL.<br> | ||
| + | Nanodrop:<br> | ||
| + | <table style="text-align: left; width: 100px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 98px;">Sample ID<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 43px;">ng/uL<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">260/280<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">260/230<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 49px;">PCR product prior | ||
| + | to dilution<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 43px;">149.93<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">0.99<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">0.38<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 49px;">PCR product after | ||
| + | dilution<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 43px;">89.76<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">0.99<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">0.35<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table><br> | ||
| + | A total of 70 microlitres is loaded onto a 2% agarose gel.<br> | ||
| + | For the GFX gel extraction 350mg of gel was used.<br> | ||
| + | For the Fermentas extraction 460mg of gel was used.<br> | ||
| + | Gel electrophoresis:<br> | ||
| + | [[Image:Team-SDU-Denmark-Gelextractiontest.jpg|300px]]<br><br> | ||
| + | ''Results:''<br> | ||
| + | <table style="text-align: left; width: 100px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Sample ID<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">ng/uL<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">260/280<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">260/230<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">GFX 1<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">13.72<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2.17<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">0.08<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">GFX2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">16.8<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2.02<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">0.05<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">GFX pooled<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">15.51<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">2.09<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">0.06<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Fermentas 1<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">20.72<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1.9<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">0.61<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Fermentas 2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">20.42<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1.86<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">0.45<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">Fermentas pooled<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">21.38<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">1.87<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">0.51<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table><br><br> | ||
| + | ''Analysis:''<br> | ||
| + | More DNA is extracted with the fermentas kit. This may be due to the short centrifugation time in the GFX protocol. Furthermore the GFX kit that was used was old and the buffers may have been contaminated.<br><br> | ||
| + | |||
== Group: Retinal == | == Group: Retinal == | ||
<br> | <br> | ||
| - | === Transformation of K081005 in pSB1A2 (constitutive promoter and RBS combined),R0011 in pSB1A2, pSB3C5 w. J04450 and pSB3T5 w. J04450 in Top 10 E.Coli === | + | === Transformation of [http://partsregistry.org/Part:BBa_K081005 K081005] in pSB1A2 (constitutive promoter and RBS combined),[http://partsregistry.org/Part:BBa_R0011 R0011] in pSB1A2, pSB3C5 w. [http://partsregistry.org/Part:BBa_J04450 J04450] and pSB3T5 w. J04450 in Top 10 E.Coli === |
Start date: 19/07 End date: 20/07<br> | Start date: 19/07 End date: 20/07<br> | ||
''Methods:'' ON culture, making competent cells, transformation <br><br> | ''Methods:'' ON culture, making competent cells, transformation <br><br> | ||
| Line 663: | Line 1,512: | ||
--[[User:Tipi|Tipi]] 08:17, 21 July 2010 (UTC)<br><br> | --[[User:Tipi|Tipi]] 08:17, 21 July 2010 (UTC)<br><br> | ||
| - | === Coloni PCR of R0011 in pSB1A2 and K081005 in pSB1A2 === | + | === Coloni PCR of [http://partsregistry.org/Part:BBa_R0011 R0011] in pSB1A2 and [http://partsregistry.org/Part:BBa_K081005 K081005] in pSB1A2 === |
Start date: 20/07 End date: 20/07<br> | Start date: 20/07 End date: 20/07<br> | ||
''Methods:'' Coloni PCR and gel electrophoresis <br><br> | ''Methods:'' Coloni PCR and gel electrophoresis <br><br> | ||
| Line 783: | Line 1,632: | ||
''Results:''<br> | ''Results:''<br> | ||
Gel electrophoresis: <br> | Gel electrophoresis: <br> | ||
| - | [[Image:Team-SDU-Denmark-Colonipcr R0011, K081005.jpg| | + | [[Image:Team-SDU-Denmark-Colonipcr R0011, K081005.jpg|300px]]<br><br> |
''Analysis:''<br> | ''Analysis:''<br> | ||
A band at app. 300bp was observed in all lanes, indicating that all 10 colonies contained the correct plasmids.<br> | A band at app. 300bp was observed in all lanes, indicating that all 10 colonies contained the correct plasmids.<br> | ||
| Line 800: | Line 1,649: | ||
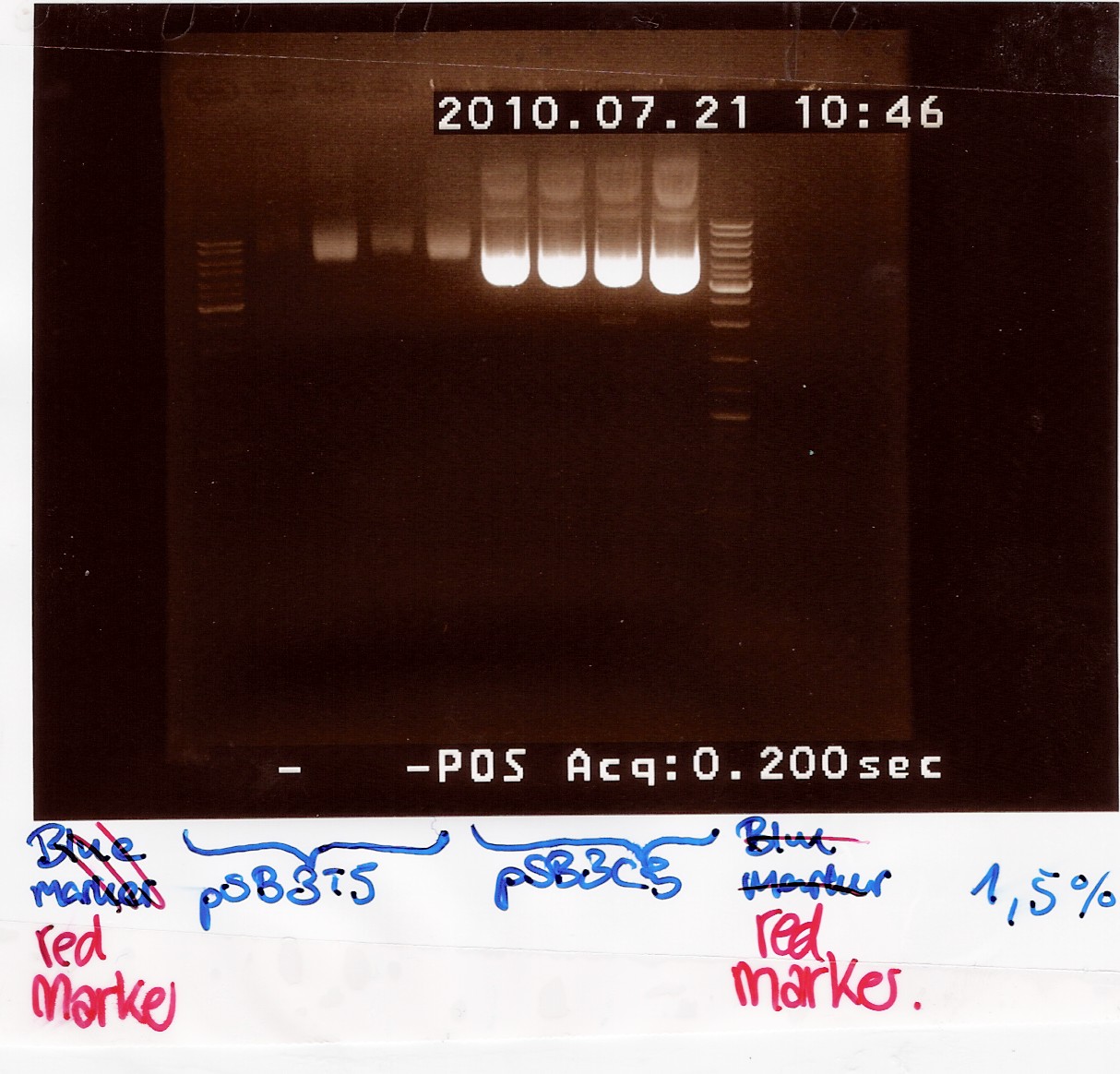
''Results:''<br> | ''Results:''<br> | ||
Gel electrophoresis: <br> | Gel electrophoresis: <br> | ||
| - | [[Image:Team-SDU-Denmark-Miniprep pSB3T5 and pSB3C5.jpg| | + | [[Image:Team-SDU-Denmark-Miniprep pSB3T5 and pSB3C5.jpg|300px]]<br> |
Nanodrop: <br> | Nanodrop: <br> | ||
| - | <table style="text-align: left; width: | + | <table style="text-align: left; width: 300px;" border="1" cellpadding="2" |
cellspacing="2"> | cellspacing="2"> | ||
<tr> | <tr> | ||
| Line 903: | Line 1,752: | ||
Nanodrop of pooled samples:<br> | Nanodrop of pooled samples:<br> | ||
| - | <table style="text-align: left; width: | + | <table style="text-align: left; width: 300px;" border="1" cellpadding="2" |
cellspacing="2"> | cellspacing="2"> | ||
<tr> | <tr> | ||
| Line 964: | Line 1,813: | ||
The gene is inserted into a pOT2 plasmid. A plasmid map can be found [http://www.fruitfly.org/about/methods/pOT2vector.html here]:<br><br> | The gene is inserted into a pOT2 plasmid. A plasmid map can be found [http://www.fruitfly.org/about/methods/pOT2vector.html here]:<br><br> | ||
Two of the chlor plates were damaged (one very slightly) and one ampicilin plate was torn up, again. (good thing two were dried). Plates were incubated ON at 37°.<BR><BR> | Two of the chlor plates were damaged (one very slightly) and one ampicilin plate was torn up, again. (good thing two were dried). Plates were incubated ON at 37°.<BR><BR> | ||
| + | Results: Colonies showed on all plates.<br><br> | ||
| + | Transformations have been succesfull. If our gene is in there will be tested next week. | ||
| + | |||
=== Coloni PCR of flhD/C in pSB1C3 === | === Coloni PCR of flhD/C in pSB1C3 === | ||
Start date: 21/07 End date: 21/07<br> | Start date: 21/07 End date: 21/07<br> | ||
| Line 971: | Line 1,823: | ||
''Notes:''<br> | ''Notes:''<br> | ||
Coloni PCR was made on flhD/C in pSB1C3 from [https://2010.igem.org/Team:SDU-Denmark/labnotes2#Transformation_of_flhD.2FC_in_pSB1C3_and_test_plasmid_in_Top_10_E.Coli Transformation].<br> | Coloni PCR was made on flhD/C in pSB1C3 from [https://2010.igem.org/Team:SDU-Denmark/labnotes2#Transformation_of_flhD.2FC_in_pSB1C3_and_test_plasmid_in_Top_10_E.Coli Transformation].<br> | ||
| - | 10 colonies from plates plated with cells transformed with L2 and L3 ligation mixtures (see [ | + | 10 colonies from plates plated with cells transformed with L2 and L3 ligation mixtures (see [https://2010.igem.org/Team:SDU-Denmark/labnotes2#Ligation_of_flhD.2FC_.28native_coding_sequence.29_and_pSB1C3 Ligation]).<br> |
Coloni 1-5 is taken from L2 plates | Coloni 1-5 is taken from L2 plates | ||
coloni 6-10 is taken from L3 plates <br> | coloni 6-10 is taken from L3 plates <br> | ||
| Line 1,090: | Line 1,942: | ||
''Results:''<br> | ''Results:''<br> | ||
Gel electrophoresis: <br> | Gel electrophoresis: <br> | ||
| - | [[Image:Team-SDU-Denmark-coloni PCR flhDC ligation.jpg| | + | [[Image:Team-SDU-Denmark-coloni PCR flhDC ligation.jpg|300px]]<br><br> |
''Analysis:''<br> | ''Analysis:''<br> | ||
Latest revision as of 19:23, 24 October 2010
 "
"