Team:Purdue/Notebook
From 2010.igem.org
m |
|||
| (20 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | {{Team:Purdue/header}} | |
| + | |||
| + | [[Image:Growth_Room.JPG|thumb]] | ||
==Notebook== | ==Notebook== | ||
| Line 6: | Line 8: | ||
===6/16/10=== | ===6/16/10=== | ||
Prepared nine pots of wildtype (Col-0) ''A. thaliana'' and moved to cold storage for stratification. Pots intended for use in ''Agrobacterium'' flower-dip. Seeds were sewn in soilless media and the pot was covered with a wide meshing to retain the soil. | Prepared nine pots of wildtype (Col-0) ''A. thaliana'' and moved to cold storage for stratification. Pots intended for use in ''Agrobacterium'' flower-dip. Seeds were sewn in soilless media and the pot was covered with a wide meshing to retain the soil. | ||
| - | + | <gallery> | |
| + | Image:Joe_Purdue.JPG | ||
| + | Image:John_Purdue.JPG | ||
| + | Image:Landon_Joe.JPG | ||
| + | </gallery> | ||
Prepared proliferative media for GBAM1 glioblastoma cell line. Began culturing cells (n = 14). | Prepared proliferative media for GBAM1 glioblastoma cell line. Began culturing cells (n = 14). | ||
| + | Got to start seeing neurospheres form from the neural stem cells. | ||
===6/19/10=== | ===6/19/10=== | ||
| Line 14: | Line 21: | ||
===6/21/10=== | ===6/21/10=== | ||
Transfered pots from cold storage to greenhouse. Prepared 0.5 MS agar media and sterilized seeds for sterile growth, to be sewn on 6/23. Seeds left in dilute agar solution in fridge to stratify. | Transfered pots from cold storage to greenhouse. Prepared 0.5 MS agar media and sterilized seeds for sterile growth, to be sewn on 6/23. Seeds left in dilute agar solution in fridge to stratify. | ||
| + | |||
| + | <gallery>Image:Pot_Close_Up.JPG</gallery> | ||
Replenished GBAM1 media. Selected cells for lactate flux sensing. | Replenished GBAM1 media. Selected cells for lactate flux sensing. | ||
| Line 20: | Line 29: | ||
Agar plates poured. Seeds to be sewn on 6/24 to give time for the media to solidify. | Agar plates poured. Seeds to be sewn on 6/24 to give time for the media to solidify. | ||
| - | + | <gallery>Image:First_Plates.JPG</gallery> | |
===6/24/10=== | ===6/24/10=== | ||
| Line 27: | Line 36: | ||
Replenished GBAM1 media. Selected cells for oxygen flux sensing. | Replenished GBAM1 media. Selected cells for oxygen flux sensing. | ||
| - | + | <gallery>Image:Failed_Plate.JPG</gallery> | |
===6/25/10=== | ===6/25/10=== | ||
| Line 48: | Line 57: | ||
Transformation of E. coli failed. | Transformation of E. coli failed. | ||
| + | |||
| + | <gallery>Image:Death_Cart.JPG</gallery> | ||
===7/1/10=== | ===7/1/10=== | ||
Poured agar. Placed new plants onto sterile plates. Placed in growth room. Placed potted plants in mist room. | Poured agar. Placed new plants onto sterile plates. Placed in growth room. Placed potted plants in mist room. | ||
| + | |||
| + | <gallery>Image:Mist_Bench.JPG</gallery> | ||
===7/6/10=== | ===7/6/10=== | ||
| Line 57: | Line 70: | ||
Successful growth of all plants noted. One small mold colony present in plate. | Successful growth of all plants noted. One small mold colony present in plate. | ||
| - | + | <gallery> | |
| + | Image:Good_Plates_Large.JPG | ||
| + | Image:Good_Plates_Small.JPG | ||
| + | </gallery> | ||
===7/12/10=== | ===7/12/10=== | ||
| Line 66: | Line 82: | ||
Transformation of E. coli: Success | Transformation of E. coli: Success | ||
| + | |||
| + | ===7/14/2010=== | ||
| + | We followed the qiagen miniprep protocol to purify the luciferase plasmid dna from the earlier successful E.coli transformation. | ||
| + | |||
| + | ===7/20/10=== | ||
| + | We made a 50 µg/mL solution of kanamycin and LB agar broth which we then used to make 21 plates for further E. coli transformation techniques. After about 30 minutes we then covered the plates in parafilm and placed them in a refrigerator. | ||
| + | |||
| + | ===7/22/2010=== | ||
| + | We added 5mL of agar soluntion and 25 micrograms of L-kanamycin to two tubes and then placed one cell colony in each of the tubes. We then placed them in the 37 degree Celsius incubator. | ||
| + | |||
| + | ===7/27/2010=== | ||
| + | We are transforming E. coli. We got Venus YFP and pSB2K3 from iGEM spring 2010 distribution kit. We got SOC broth (about 450 mL) and added 8 µL of E.coli and 2 µL of plasmid to each tube. We heatshocked the E. coli at around 39.5 degrees Celsius not at the usual 42 degrees Celsius. The plasmids contained YFP, luciferase, and psb2k3. We also had one control group for a total of four tubes. We had a total volume of a little over 200 µL. We added about 50 µL of E. coli to each plate and streaked. | ||
| + | |||
| + | ===7/28/2010=== | ||
| + | We checked the E. coli growth from yesterday’s transformations. The control group had E. coli growth which it shouldn’t have because it should have no resistance to ampicillin in the plates. The YFP group had no growth at all and the plasmid vector psb2k3 group had spotty growth almost like only places where it had been streaked. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | <gallery> | ||
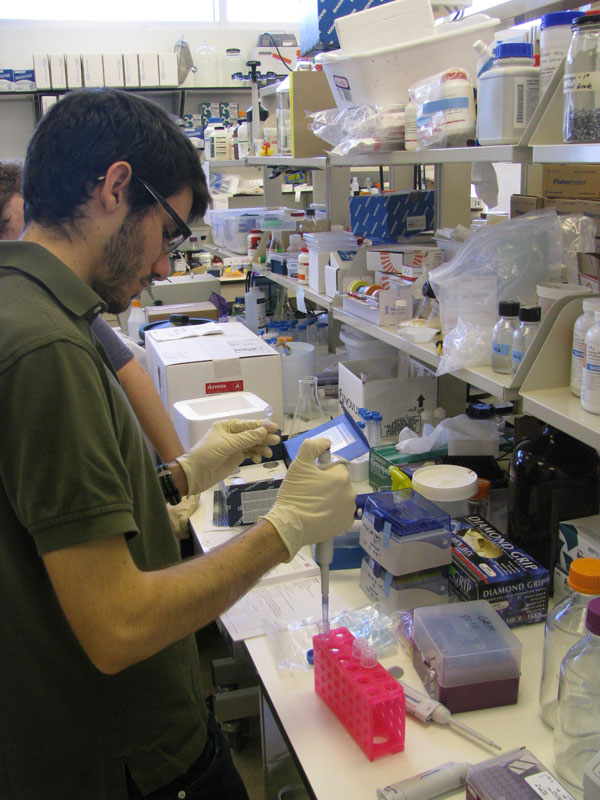
| + | Image:Sean2.jpg|Sean pipeting during MiniPrep | ||
| + | Image:Sean_John.jpg|John and Sean debate next step in transformation | ||
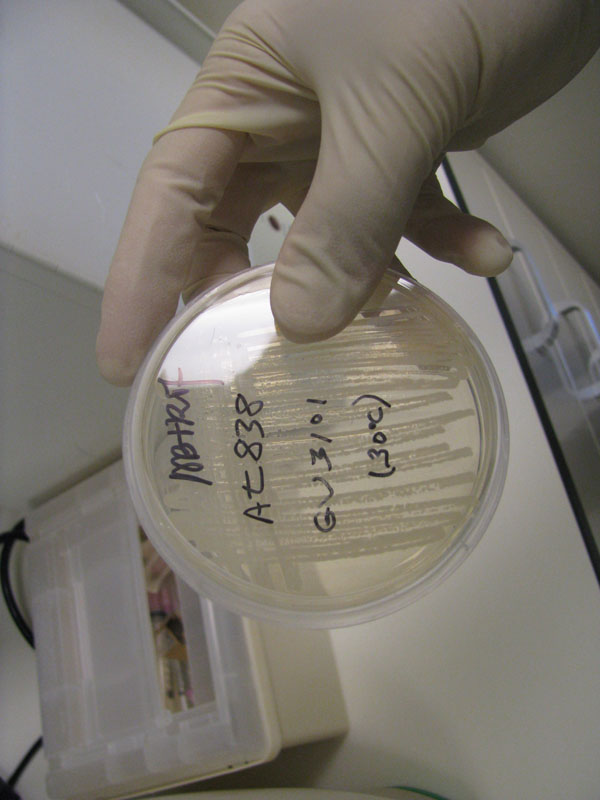
| + | Image:Agro.jpg|Agrobacterium strain | ||
| + | Image:Agro_Chamber.jpg|Agro stored in 30 C incubator | ||
| + | Image:Landon.jpg|Landon studies the ager | ||
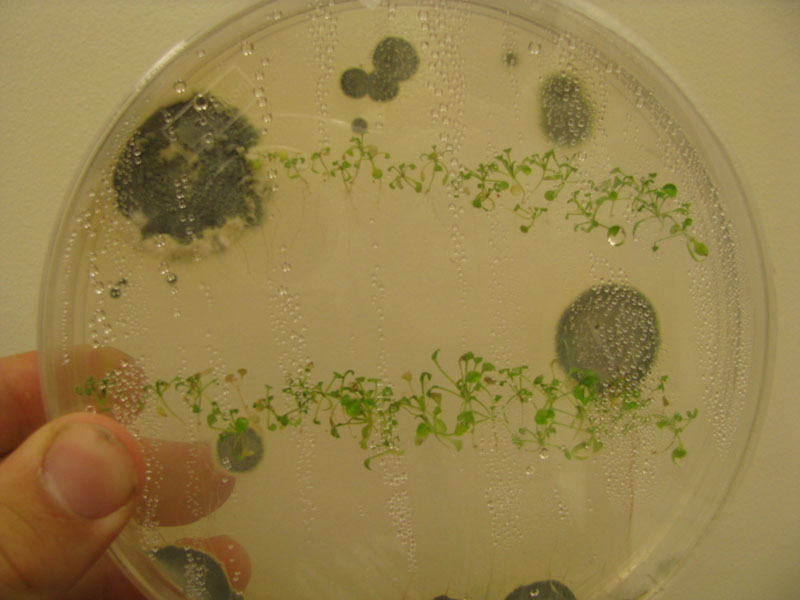
| + | Image:Bad.JPG|Contamination of arabidopsis plate | ||
| + | </gallery> | ||
Latest revision as of 15:38, 11 September 2010
Home
The Team
The Project
Parts Submitted to the Registry
Modeling
Notebook
Safety
Collaboration
Contents |
Notebook
6/16/10
Prepared nine pots of wildtype (Col-0) A. thaliana and moved to cold storage for stratification. Pots intended for use in Agrobacterium flower-dip. Seeds were sewn in soilless media and the pot was covered with a wide meshing to retain the soil.
Prepared proliferative media for GBAM1 glioblastoma cell line. Began culturing cells (n = 14). Got to start seeing neurospheres form from the neural stem cells.
6/19/10
Divided GBAM1 cells into media for proliferation and differentiation.
6/21/10
Transfered pots from cold storage to greenhouse. Prepared 0.5 MS agar media and sterilized seeds for sterile growth, to be sewn on 6/23. Seeds left in dilute agar solution in fridge to stratify.
Replenished GBAM1 media. Selected cells for lactate flux sensing.
6/23/10
Agar plates poured. Seeds to be sewn on 6/24 to give time for the media to solidify.
6/24/10
Seeds sown onto agar. Five large culture plates and two small plates were then transfered into horticulture growth chamber. Seeds in soil media forecasted to germinate by 6/26.
Replenished GBAM1 media. Selected cells for oxygen flux sensing.
6/25/10
A. thaliana expirement mapping: Testing low oxygen response in WT and Transgenic using atmospheric chamber in Purdue Agronomy department. Using sensors to measure ethanol, oxygen, auxin response in control and in hypoxic conditions.
6/25/10
Communicating with IU iGEM team concerning synergies including arabidopsis transformation workshop.
Communicating with Naperville, Ill high school student as part of community outreach experience.
Planning Purdue University campus poster session to involve public in forum discussion on genetic engineering.
6/28/10
Sterilized seeds for plates. Potted seeds on soil, placed in cold room.
Transformed E. coli heat shock method with <partinfo>Bba_I712019</partinfo> - firefly luciferase.
6/29/10
Transformation of E. coli failed.
7/1/10
Poured agar. Placed new plants onto sterile plates. Placed in growth room. Placed potted plants in mist room.
7/6/10
Successful growth of all plants noted. One small mold colony present in plate.
7/12/10
Transformation of E. coli: attempt # 2 <partinfo>Bba_I712019</partinfo>
7/13/10
Transformation of E. coli: Success
7/14/2010
We followed the qiagen miniprep protocol to purify the luciferase plasmid dna from the earlier successful E.coli transformation.
7/20/10
We made a 50 µg/mL solution of kanamycin and LB agar broth which we then used to make 21 plates for further E. coli transformation techniques. After about 30 minutes we then covered the plates in parafilm and placed them in a refrigerator.
7/22/2010
We added 5mL of agar soluntion and 25 micrograms of L-kanamycin to two tubes and then placed one cell colony in each of the tubes. We then placed them in the 37 degree Celsius incubator.
7/27/2010
We are transforming E. coli. We got Venus YFP and pSB2K3 from iGEM spring 2010 distribution kit. We got SOC broth (about 450 mL) and added 8 µL of E.coli and 2 µL of plasmid to each tube. We heatshocked the E. coli at around 39.5 degrees Celsius not at the usual 42 degrees Celsius. The plasmids contained YFP, luciferase, and psb2k3. We also had one control group for a total of four tubes. We had a total volume of a little over 200 µL. We added about 50 µL of E. coli to each plate and streaked.
7/28/2010
We checked the E. coli growth from yesterday’s transformations. The control group had E. coli growth which it shouldn’t have because it should have no resistance to ampicillin in the plates. The YFP group had no growth at all and the plasmid vector psb2k3 group had spotty growth almost like only places where it had been streaked.
 "
"