Team:Brown/Project/Light pattern/Future
From 2010.igem.org
(→Future Directions) |
|||
| (8 intermediate revisions not shown) | |||
| Line 3: | Line 3: | ||
{{:Team:Brown/templates/Lpmenu}} | {{:Team:Brown/templates/Lpmenu}} | ||
==Future Directions== | ==Future Directions== | ||
| + | Ideally, we would like to construct our circuit in entirety, and test its function in the laboratory, not just through mathmatical modeling. Furthermore, there are a couple areas where modeling and reexamination of our project goals has led us to believe we can make improvements in the design of the circuit. Specifically, the circuit now has two promoters, pLac/Mnt and AraC+pBad that require some chemical (IPTG and arabinose) to be present in the culture. While our current circuit design still enables us to show proof of concept, that we can switch between the states by changing only the presence of light, our ultimate goal is to reduce cost of manufacturing processes and increase the efficiency of drug synthesis. Because, as explained in the [[:Team:Brown/Project/Light_pattern/Overview| Overview section]], large reactions that rely on chemical induction, even in some part, can be extremely expensive (on account of the chemicals themselves and purification), we would prefer not to use the pLac/Mnt nor pBad promoters. Thus, perhaps in the future the circuit could be slightly reworked to avoid use of this type of promoter. | ||
| - | + | Quite far down the road is the possibility of directed evolution of specific components of the circuit. As the modeling suggests, given analysis of sensitivity, certain parameters of components of the circuit have a large effect on the overall distinctness of states and time-frame with which they occur. Directed evolution could be utilized to fine tune the characteristics of individual components, thus achieving a better circuit. | |
| + | |||
| + | Given a fully successful and optimized circuit, we believe our approach has the potential to greatly cut manufacturing costs, making medicine more affordable, biofuel processes more reasonable, etc. We included below a couple example manufacturing processes that our circuit could possibly be applied to. | ||
| + | |||
| + | |||
| + | Applications: | ||
| + | |||
| + | |||
| + | The four-state Light-Pattern Controlled Circuit has many useful applications to manufacturing of end products which require several enzymatic steps. With the output of each state in the circuit as the enzyme of the subsequent step in the manufacturing process, the synthesis of many compounds may be combined to a one-pot biosynthetic process, significantly cutting costs and risk of contamination. Another advantage of applying the light-pattern controlled circuit is in drawing manufacturers towards the greener biosynthetic processes; this will cutting down on toxic waste that would have accumulated in alternative chemical synthetic processes, which right now yields 25-100 kg of waste per kilogram of product in the average pharmaceutical synthesis (Sheldon, 1997)! | ||
| + | |||
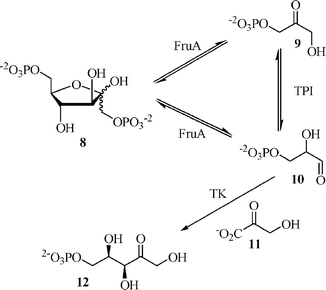
| + | The field of enzyme engineering is relatively new and has a great deal of potential for growth Already, there are examples of multistep enzyme-catalyzed processes to which our light-pattern controlled circuit can be applied. An example of this is the synthesis of D-xylulose 5-phosphate synthesis developed by Zimmermann which makes it possible to utilize both triose phosphate equivalents that are formed from aldolase-catalyzed fructose diphosphate cleavage (Zimmermann, 1999). | ||
| + | |||
| + | [[Image:4.gif]] | ||
| + | |||
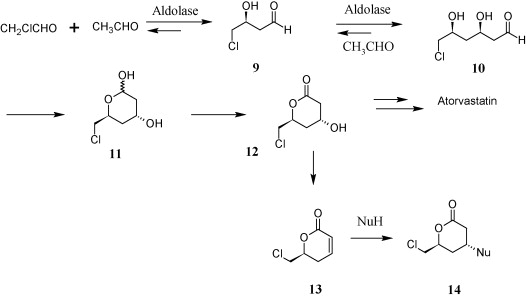
| + | A current limitation of one-pot biosynthesis using transgenic bacteria is the need for site-isolation, the physical separation of catalysts from each other (Broadwater, 2005). With our circuit, this roadblock may be partially remedied by temporal isolation. With this comes the possibility of combining currently separated biosynthetic processes into a one-pot synthesis. An example of such a possibility may be in the production of the top-selling drug in the world, Atorvastatin (Lipitor), in which many intermediates can be made through biocatalysts such as alcohol dehydrogenase, aldolase, nitrilase, lipase, halohydrin dehalogenase (Patel, 2009), as shown below: | ||
| + | |||
| + | Alcohol Dehydrogenase: | ||
| + | |||
| + | [[Image:App1.jpg]] | ||
| + | |||
| + | Aldoase: | ||
| + | |||
| + | [[Image:App2.jpg]] | ||
| + | |||
| + | |||
| + | Citations: | ||
| + | |||
| + | S. J. Broadwater, S. L. Roth, K. E. Price, M. Kobašlija, D. T. McQuade, Org. Biomol. Chem. 2005, 3, 2899–2906; | ||
| + | |||
| + | J.M. Patel, J. Mol. Catal. B: Enzym. 61 (2009), pp. 123–128. | ||
| + | |||
| + | R. A. Sheldon, J. Chem. Technol. Biotechnol., 1997, 68, 381–388 | ||
| + | |||
| + | F.T. Zimmermann, A. Schneider, U. Schörken, G.A. Sprenger and W.-D. Fessner, Tetrahedron: Asymmetry 10 (1999), pp. 1643–1646. | ||
Latest revision as of 00:21, 28 October 2010
Light-Pattern Controlled Circuit
Future Directions
Ideally, we would like to construct our circuit in entirety, and test its function in the laboratory, not just through mathmatical modeling. Furthermore, there are a couple areas where modeling and reexamination of our project goals has led us to believe we can make improvements in the design of the circuit. Specifically, the circuit now has two promoters, pLac/Mnt and AraC+pBad that require some chemical (IPTG and arabinose) to be present in the culture. While our current circuit design still enables us to show proof of concept, that we can switch between the states by changing only the presence of light, our ultimate goal is to reduce cost of manufacturing processes and increase the efficiency of drug synthesis. Because, as explained in the Overview section, large reactions that rely on chemical induction, even in some part, can be extremely expensive (on account of the chemicals themselves and purification), we would prefer not to use the pLac/Mnt nor pBad promoters. Thus, perhaps in the future the circuit could be slightly reworked to avoid use of this type of promoter.
Quite far down the road is the possibility of directed evolution of specific components of the circuit. As the modeling suggests, given analysis of sensitivity, certain parameters of components of the circuit have a large effect on the overall distinctness of states and time-frame with which they occur. Directed evolution could be utilized to fine tune the characteristics of individual components, thus achieving a better circuit.
Given a fully successful and optimized circuit, we believe our approach has the potential to greatly cut manufacturing costs, making medicine more affordable, biofuel processes more reasonable, etc. We included below a couple example manufacturing processes that our circuit could possibly be applied to.
Applications:
The four-state Light-Pattern Controlled Circuit has many useful applications to manufacturing of end products which require several enzymatic steps. With the output of each state in the circuit as the enzyme of the subsequent step in the manufacturing process, the synthesis of many compounds may be combined to a one-pot biosynthetic process, significantly cutting costs and risk of contamination. Another advantage of applying the light-pattern controlled circuit is in drawing manufacturers towards the greener biosynthetic processes; this will cutting down on toxic waste that would have accumulated in alternative chemical synthetic processes, which right now yields 25-100 kg of waste per kilogram of product in the average pharmaceutical synthesis (Sheldon, 1997)!
The field of enzyme engineering is relatively new and has a great deal of potential for growth Already, there are examples of multistep enzyme-catalyzed processes to which our light-pattern controlled circuit can be applied. An example of this is the synthesis of D-xylulose 5-phosphate synthesis developed by Zimmermann which makes it possible to utilize both triose phosphate equivalents that are formed from aldolase-catalyzed fructose diphosphate cleavage (Zimmermann, 1999).
A current limitation of one-pot biosynthesis using transgenic bacteria is the need for site-isolation, the physical separation of catalysts from each other (Broadwater, 2005). With our circuit, this roadblock may be partially remedied by temporal isolation. With this comes the possibility of combining currently separated biosynthetic processes into a one-pot synthesis. An example of such a possibility may be in the production of the top-selling drug in the world, Atorvastatin (Lipitor), in which many intermediates can be made through biocatalysts such as alcohol dehydrogenase, aldolase, nitrilase, lipase, halohydrin dehalogenase (Patel, 2009), as shown below:
Alcohol Dehydrogenase:
Aldoase:
Citations:
S. J. Broadwater, S. L. Roth, K. E. Price, M. Kobašlija, D. T. McQuade, Org. Biomol. Chem. 2005, 3, 2899–2906;
J.M. Patel, J. Mol. Catal. B: Enzym. 61 (2009), pp. 123–128.
R. A. Sheldon, J. Chem. Technol. Biotechnol., 1997, 68, 381–388
F.T. Zimmermann, A. Schneider, U. Schörken, G.A. Sprenger and W.-D. Fessner, Tetrahedron: Asymmetry 10 (1999), pp. 1643–1646.
 "
"