Team:Heidelberg/Notebook/Homology Based/October
From 2010.igem.org
(→22/10/2010) |
Philippbayer (Talk | contribs) (→22/10/2010) |
||
| (2 intermediate revisions not shown) | |||
| Line 231: | Line 231: | ||
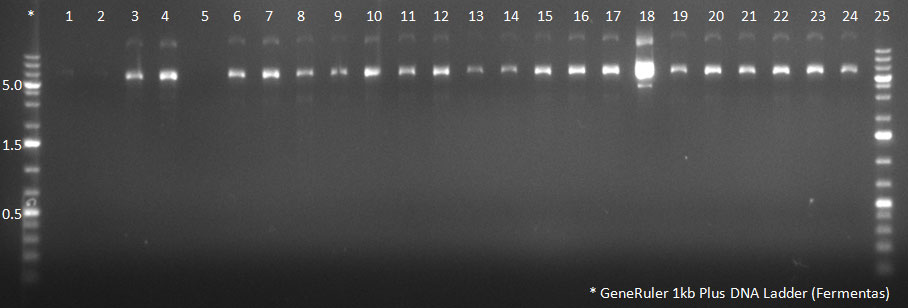
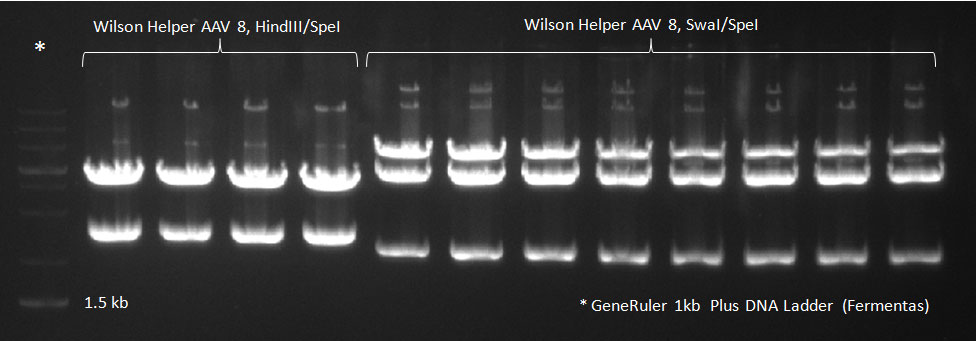
The conditions for the PCR were the same as the second PCR for amplification of the shuffled capsids (see above). The bands for cap genes (2.2 KB) were purified from the gel, then digested with SwaI and SpeI, together with Wilson wild-type vector (standard 20 microliter digestion in NEB buffer 4, for 2 hours at 25 C). Qiagen nucleotide removal followed for the cap genes, and the vector was gel-purified. A ligation reaction of cap genes into the vector was carried out with NEB Quick Ligase (according to the standard manufacturer's recommendations), for 1 hour 30 min at room temperature. | The conditions for the PCR were the same as the second PCR for amplification of the shuffled capsids (see above). The bands for cap genes (2.2 KB) were purified from the gel, then digested with SwaI and SpeI, together with Wilson wild-type vector (standard 20 microliter digestion in NEB buffer 4, for 2 hours at 25 C). Qiagen nucleotide removal followed for the cap genes, and the vector was gel-purified. A ligation reaction of cap genes into the vector was carried out with NEB Quick Ligase (according to the standard manufacturer's recommendations), for 1 hour 30 min at room temperature. | ||
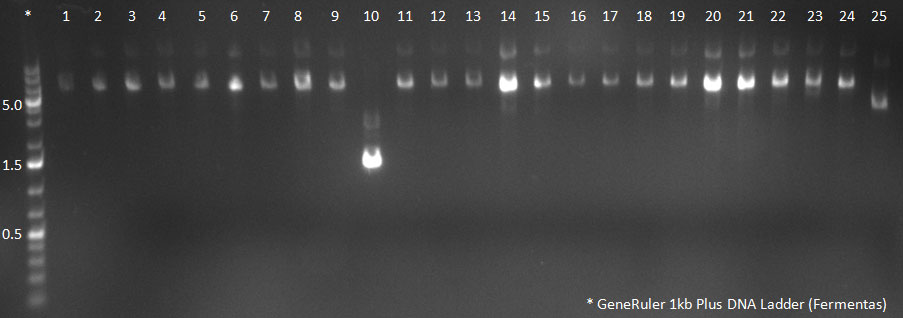
| + | [[Image:102310_20clones.gif|300px]] | ||
| + | PCR for Huh-7 clones | ||
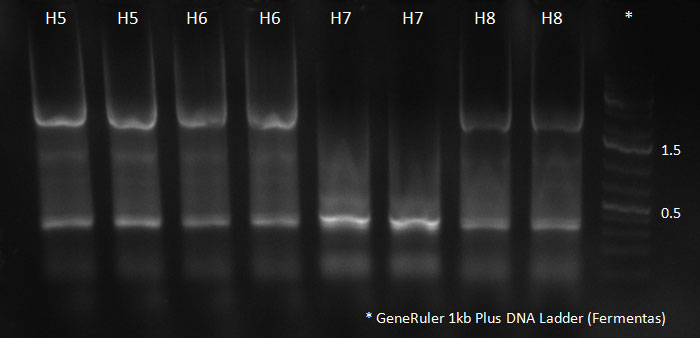
[[Image:102310_H5-8_supern_PCR.jpg|300px]] | [[Image:102310_H5-8_supern_PCR.jpg|300px]] | ||
| Line 253: | Line 255: | ||
==25/10/2010== | ==25/10/2010== | ||
| - | * mini-preps were run for the samples from the previous day, and triple transfection of the Wilson construct with cap genes, Adeno-helper plasmid and a YFP construct with ITRs was done on 4 24-well plates of Hek293 cells in replica, each well containing an AAV construct from a different selection round on Huh-7 cells with different conditions during the selection process (washing after 30 min of AAV addition or no washing, see above). | + | * mini-preps were run for the samples from the previous day, and triple transfection of the Wilson construct with cap genes, Adeno-helper plasmid and a YFP construct with ITRs was done on 4 24-well plates of Hek293 cells in replica, each well containing an AAV construct from a different selection round on Huh-7 cells with different conditions during the selection process (washing after 30 min of AAV addition or no washing, see above). |
| + | |||
| + | From each selection round 12 samples were transfected, plus AAV wildtypes 1-12. | ||
==26/10/2010== | ==26/10/2010== | ||
* The AAVs were harvested from the cells of one set of the plates (see previous day) using freeze-thaw cycles, and 20 microliters of the supernatant after centrifugation were used to infect HeLa, Huh-7 and primary hepatocyte cells in a 96-well plate format. This was done in replicates. The plates are then used for FACS analysis of YFP expression. | * The AAVs were harvested from the cells of one set of the plates (see previous day) using freeze-thaw cycles, and 20 microliters of the supernatant after centrifugation were used to infect HeLa, Huh-7 and primary hepatocyte cells in a 96-well plate format. This was done in replicates. The plates are then used for FACS analysis of YFP expression. | ||
| + | |||
| + | * The clones picked were sent for sequencing. | ||
==27/10/2010== | ==27/10/2010== | ||
Latest revision as of 03:56, 28 October 2010

October01/10/2010
02/10/2010
03/10/2010
05/10/2010
- Washing the flasks with the medium they contain, and collecting the media from 13 flasks in 500 ml corning conical centrifuge tubes. - The cells were pelleted by centrifuging at 1500 rpm for 15 min at 4 ͦC - Discard supernatant - Washing: Resuspend the cells in 1X PBS and transfer to a 50 ml falcon - Pellet the cells again by centrifuging at 1500 rpm for 15 min at 4 ͦC - Discard supernatant
- Add 20 ml lysis buffer to the cell pellet - Put in liquid nitrogen for 5 min - Thaw at 37ͦC The cycles were repeated for 5 times, and the sample was frozen for next day. 06/10/2010
- The sample from previous day was sonicated in a sonication bath for 1 min 20 sec. - 50 units/ml benzonase were added to destroy RNA and DNA from non-AAV sources, which could later interfere with qPCR. - The sample was incubated at 37 ͦC for 30 min, and vortexed every 10 min. - The sample was then centrifuged for 15 min at 3270 xg - The supernatant was transferred into a new 50 ml falcon
- A Pasteur pipette was plugged into a Beckman quick-seal centrifuge tube - Using a 1000 µl pipette, 20 ml of the virus suspension were added the gradient was poured through the Pasteur pipette in the following order: - 7 ml 15% Iodixanol solution - 5 ml 25% Iodixanol solution - 4 ml 40% Iodixanol solution - 4 ml 60% Iodixanol solution - The Pasteur pipette was carefully removed and the tube was sealed. A tare tube was prepared in the same way. - Ultracentrifugation of the sample was carried out at 50,000 rpm for 2:30 hours at 4ͦC. - The virus-containing phase is the 40% iodixanol phase, which was sucked out using a syringe and a needle. The AAV sample was divided into aliquotes, one of them stored at 4 ͦC for qPCR the next day, and the rest were frozen in liquid nitrogen then transferred to -20 ͦC. 07/10/2010
- AAV MOI 100, 10 and 1 were used on Huh-7 cells plated on 6-well plates, and the viruses were allowed to infect cells for 1 hour and 30 min. - Adeno-virus 5 (MOI 10) was added then in a Biohazard II lab under the superviosion of lab personnel. - Cells were incubated in the Biohazard II lab. 08/10/2010- A non-ITR AAV helper vector was obtained and maxi-preped to clone into it the cap genes from the 50 single clones picked. 09/10/2010- The non-ITR vector was cut with AscI and PacI, and so was the pTR-UF3 containg cap genes from the 50 clones. The bands were purified from the gel.
10/10/2010
11/10/2010
13/10/2010
14/10/2010
- two 24-well plates HepG2 cells - Two 24-well plates Huh-7 cells - Two 24-well plates primary hepatocytes 15/10/2010
16/10/2010
Clone #43 was later injected into mice with a luc2-SV40 construct. 17/10/2010
18/10/2010
19/10/2010
21/10/2010
22/10/2010
The conditions for the PCR were the same as the second PCR for amplification of the shuffled capsids (see above). The bands for cap genes (2.2 KB) were purified from the gel, then digested with SwaI and SpeI, together with Wilson wild-type vector (standard 20 microliter digestion in NEB buffer 4, for 2 hours at 25 C). Qiagen nucleotide removal followed for the cap genes, and the vector was gel-purified. A ligation reaction of cap genes into the vector was carried out with NEB Quick Ligase (according to the standard manufacturer's recommendations), for 1 hour 30 min at room temperature. 300px PCR for Huh-7 clones 23/10/2010
24/10/2010
25/10/2010
From each selection round 12 samples were transfected, plus AAV wildtypes 1-12. 26/10/2010
27/10/2010
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"