Team:TU Munich/Parts
From 2010.igem.org
Hartlmueller (Talk | contribs) (→Parts Overview) |
Hartlmueller (Talk | contribs) |
||
| (17 intermediate revisions not shown) | |||
| Line 6: | Line 6: | ||
<!-- ############## WIKI-PAGE STARTS HERE ############## --> | <!-- ############## WIKI-PAGE STARTS HERE ############## --> | ||
| - | + | =Submitted Parts= | |
| - | + | ||
| - | + | ||
| - | + | ||
<center> | <center> | ||
<groupparts align="center" width="100%">igem2010 TU_Munich</groupparts> | <groupparts align="center" width="100%">igem2010 TU_Munich</groupparts> | ||
| Line 15: | Line 12: | ||
<br> | <br> | ||
| - | |||
| - | ==Malachite Green Binding Aptamer - BBa_K494000== | + | ==Malachite Green Binding Aptamer - <partinfo>BBa_K494000</partinfo>== |
<br> | <br> | ||
Methods to visualize nucleic acids via fluorescence are rare, partly due to the size of fluorescent reporters. Thus, we present the malachite green-binding aptamer to the partsregistry. By adding only 38 bp, fluorescent determination of specific nucleic acids becomes possible allowing evalutation of PoPS-based devices via in vitro transcription.<sup>[[Team:TU_Munich/Parts#ref1|[1]]]</sup> Binding of triphenyl dye malachite green to the aptamer increases fluorescence by 2360-fold. This leads to an significant increase and a shift in absorbance from 618 to 630 nm. With an emission maximum at 652 nm, aptamer-bound malachite green fluoresces at longer wavelength than most dyes and does not interfere with those.<sup>[[Team:TU_Munich/Parts#ref2|[2]]]</sup> We provide this part for efficient ''in vitro'' evaluation of PoPS-based devices in general and switches based on our concept in particular. | Methods to visualize nucleic acids via fluorescence are rare, partly due to the size of fluorescent reporters. Thus, we present the malachite green-binding aptamer to the partsregistry. By adding only 38 bp, fluorescent determination of specific nucleic acids becomes possible allowing evalutation of PoPS-based devices via in vitro transcription.<sup>[[Team:TU_Munich/Parts#ref1|[1]]]</sup> Binding of triphenyl dye malachite green to the aptamer increases fluorescence by 2360-fold. This leads to an significant increase and a shift in absorbance from 618 to 630 nm. With an emission maximum at 652 nm, aptamer-bound malachite green fluoresces at longer wavelength than most dyes and does not interfere with those.<sup>[[Team:TU_Munich/Parts#ref2|[2]]]</sup> We provide this part for efficient ''in vitro'' evaluation of PoPS-based devices in general and switches based on our concept in particular. | ||
| Line 26: | Line 22: | ||
==Plasmids== | ==Plasmids== | ||
| - | In general we want to provide a new principle of gene regulation which can be further developed, tested and | + | In general we want to provide a new principle of gene regulation which can be further developed, tested and optimized by everybody. Therefore we focus on providing the parts needed for verification and testing of new individual switches. We provide a plasmid backbone which can be used for further cloning, a positive control to test the general functionality and measurements setups and the constructs we characterized for comparison. |
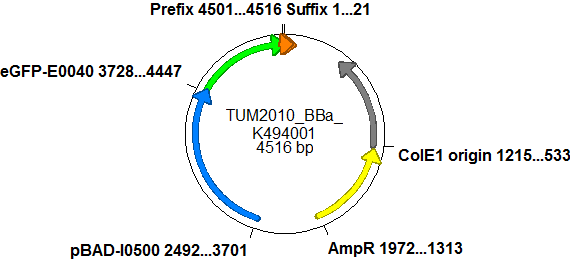
| - | ===Screening system: Backbone BBa_K494001=== | + | ===Screening system: Backbone <partinfo>BBa_K494001</partinfo>=== |
| - | + | Backbone <partinfo>BBa_K494001</partinfo> is a new backbone which can be utilized not only for evaluation of terminator-based switches but also for testing basically all PoPS devices. In principle, <partinfo>BBa_K494001</partinfo> is a modified version of <partinfo>pSB1A10</partinfo>, which allows more flexibility in regard of the reporter protein and suits very well for testing and screening of switches based on bioLOGIC principles ''in vivo'' and with an ''in vitro'' translation assay. As an internal control, eGFP is integrated into the backbone to evaluate measurement setups and induction quality. eGFP is under the control of an pBAD promoter, therefore the whole pBAD promoter cassette being also located on <partinfo>BBa_K494001</partinfo>. In comparison to <partinfo>pSB1A10</partinfo> a new cloning site was created right after the eGFP protein which allows the insertion of every DNA encoded BioBrick reporter. Reporter proteins may be exchanged to fit the challenge and can be easily exchanged if not well-functioning. | |
{{:Team:TU Munich/Templates/ToggleBoxStart}} | {{:Team:TU Munich/Templates/ToggleBoxStart}} | ||
| + | {| style="background-color:transparent" | ||
| + | |- | ||
| + | |[[Image:TUM2010 BBa K494001.png|300px|left]] | ||
| + | |For efficient usage of this part, several cloning steps must occur. First of all, the second reporter protein needs to be chosen. This can be directly introduced into the backbone using the standard BioBrick restriction enzymes EcoRI and PstI and it was done in the case of <partinfo>BBa_K494002</partinfo>. | ||
| + | |} | ||
| + | <!-- | ||
The improved screening system is optimized for the evaluation of PoPS-based devices in fluorescence measurements. RFP which was known to contain an RNase restriction site was replaced by mCherry which combines good expression yield, short maturation times and an acceptable and well-characterized quantum yield. A unique challenge for the characterization of our switches is the expression of a corresponding signal independent of the input/output measurement. Thus, we moved the BioBrick cloning site resulting in the fluorescent reporter being inside the cloning site and giving the possibility to clone independent parts behind the reporter protein. | The improved screening system is optimized for the evaluation of PoPS-based devices in fluorescence measurements. RFP which was known to contain an RNase restriction site was replaced by mCherry which combines good expression yield, short maturation times and an acceptable and well-characterized quantum yield. A unique challenge for the characterization of our switches is the expression of a corresponding signal independent of the input/output measurement. Thus, we moved the BioBrick cloning site resulting in the fluorescent reporter being inside the cloning site and giving the possibility to clone independent parts behind the reporter protein. | ||
| - | To fully function our screening plasmid need the arabinose inducible promoter BBa_I13453 in front of the PoPS-based device to screen. Using a second arabinose inducible promoter, | + | To fully function our screening plasmid need the arabinose inducible promoter BBa_I13453 in front of the PoPS-based device to screen. Using a second arabinose inducible promoter, we were able to keep eGFP as an internal standard for the tunable input via the Pbad arabinose-inducible induction system. The two identical promoters ensure the same rate of induction for eGFP and the tested PoPS-based device. Thus, obtaining comparable screening results is easy. Unfortunately, this design implicates a minor disadvantage. Two cloning steps are needed to gain an functional construct for testing any PoPS-based device.--> |
{{:Team:TU Munich/Templates/ToggleBoxEnd}} | {{:Team:TU Munich/Templates/ToggleBoxEnd}} | ||
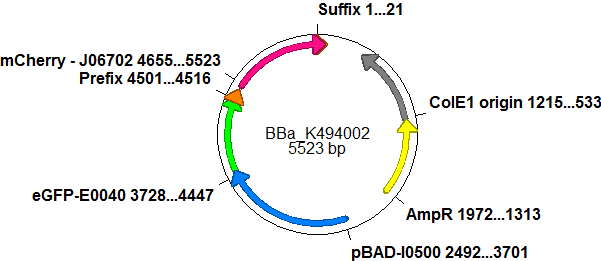
| - | ===Screening System: Positive Control | + | ===Screening System: Positive Control <partinfo>BBa_K494002</partinfo>=== |
| - | + | BBa_K494002 is the backbone <partinfo>BBa_K494001</partinfo> with an mCherry inserted into the standard BioBrick cloning site using EcoRI and PstI. mCherry was further equipped with an arabinose inducible promoter (pBAD) for good expression yield and can be used as a further reference and internal standard to compare measurements and setups. With the mCherry fluorescence in the red, a fast maturation time and a satisfying quantum yield, mCherry is the perfect complement to eGFP if fluroscence is used as an output. mCherry is expressed together with eGFP after induction with arabinose without delaying elements in between both proteins. <br> | |
| - | + | With mCherry inserted as a reporter protein, further cloning can proceed. This is another advantage of the <partinfo>BBa_K494001</partinfo> backbone: Despite the nessity of more than one cloning step, it does not cost you more time than conventional cloning strategies since a positive is generated on the way. Since the reporter protein was inserted according to BioBrick standards, additional cloning can be done using the restriction sites for EcoRI and XhoI upstream of the promoter protein and SpeI and PstI downstream of the promoter protein. This was done in the case of <partinfo>BBa_K494003</partinfo>. | |
| - | < | + | |
{{:Team:TU Munich/Templates/ToggleBoxStart}} | {{:Team:TU Munich/Templates/ToggleBoxStart}} | ||
| + | Here we present a ready to use composite part consisting of our screening system <partinfo>pSB1A10</partinfo>mod <partinfo>BBa_K494001</partinfo>, the PBad Promotor <partinfo>BBa_I13453</partinfo> and the reporter protein mCherry <partinfo>BBa_J06702</partinfo> including RBS and double Terminator <partinfo>BBa_B0015</partinfo>. This part belongs to the screening system and is intended as positive control.<br> | ||
| + | <br> | ||
| + | [[Image:TUM2010_PicPosControl3cInd.png|70px|left|]] | ||
| + | |||
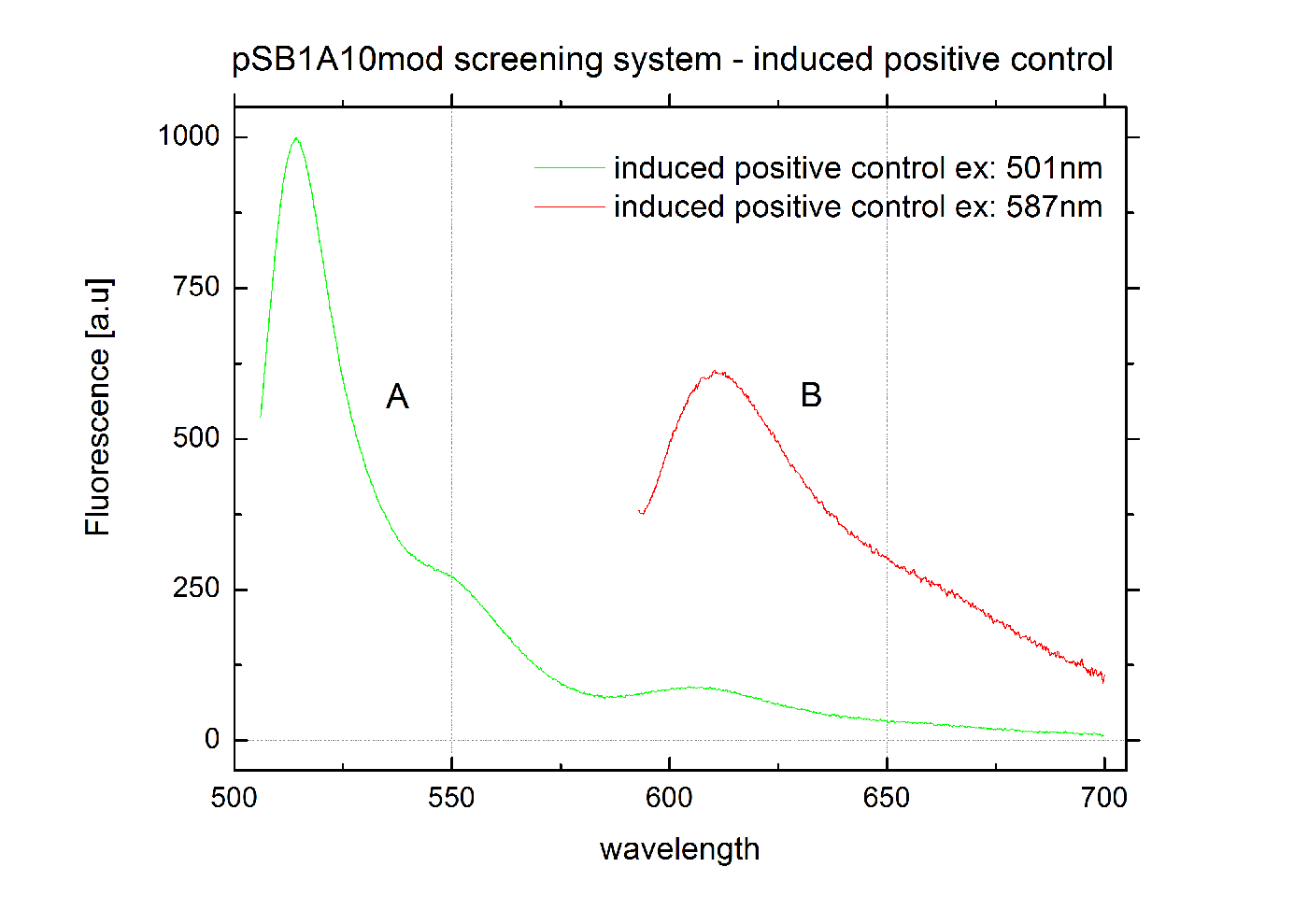
[[Image:TUM2010_graphPosControl3cInd.png|400px|thumb|center|Emission spectra of induced pSB1A10mod positive control BBa_K494002 ; A: eGFP fluorescence ex: 501 nm, B: mCherry fluorescence ex: 587 nm]] | [[Image:TUM2010_graphPosControl3cInd.png|400px|thumb|center|Emission spectra of induced pSB1A10mod positive control BBa_K494002 ; A: eGFP fluorescence ex: 501 nm, B: mCherry fluorescence ex: 587 nm]] | ||
| + | [[Image:TUM2010 BBa K494002.png|left|500px]] <br><br><br><br><br><br><br><br> | ||
{{:Team:TU Munich/Templates/ToggleBoxEnd}} | {{:Team:TU Munich/Templates/ToggleBoxEnd}} | ||
| + | <br><br> | ||
| + | ===Screening System: <partinfo>BBa_K494003</partinfo> - exemplary insert into <partinfo>BBa_K494001</partinfo>=== | ||
| + | With the reporter protein inserted, EcoRI and XhoI upstream of the chosen reporter protein and SpeI and PstI downstream the reporter protein are now free for insertion of further proteins. In the case of <partinfo>BBa_K494003</partinfo>, <partinfo>BBa_K494001</partinfo> was utilized to evaluate a terminator for both termination efficiency and evaluation of antitermination. To test the termination efficiency, a terminator was inserted upstream of the reporter protein mCherry, in this case a Rho-independent terminator based on the regulative hairpin in front of the ''his-operon'' from ''Salmonella enterica''. With this strong terminator right before mCherry, no mCherry fluorescence can be detected anymore while the eGFP fluorescences grows steadily over time. Another example of the same principle is shown in <partinfo>BBa_K494005</partinfo>. A further part can be inserted following the reporter protein using SpeI and PstI, this was done in the case of <partinfo>BBa_K494004</partinfo>. | ||
| - | |||
| - | |||
{{:Team:TU Munich/Templates/ToggleBoxStart}} | {{:Team:TU Munich/Templates/ToggleBoxStart}} | ||
| - | [[Image:TUM2010_HisTermInd.png|400px|thumb|center|Emission spectra of induced | + | Here we present a ready to use composite part consisting of our screening system <partinfo>pSB1A10</partinfo>mod <partinfo>BBa_K494001</partinfo>, the PBad Promotor BBa_I13453, the His Terminator and the reporter protein mCherry BBa_J06702 including RBS and double Terminator BBa_B0015. This part belongs to the screening system and is intended as negative control. |
| + | [[Image:TUM2010_HisTermInd.png|400px|thumb|center|Emission spectra of induced <partinfo>pSB1A10</partinfo>mod HisTerm <partinfo>BBa_K494004</partinfo> ; A: eGFP fluorescence ex: 501 nm, B: mCherry fluorescence ex: 587 nm]] | ||
{{:Team:TU Munich/Templates/ToggleBoxEnd}} | {{:Team:TU Munich/Templates/ToggleBoxEnd}} | ||
| - | ===Screening System: | + | ===Screening System: <partinfo>BBa_K494004</partinfo> - exemplary insert into <partinfo>BBa_K494001</partinfo>=== |
| - | + | BBa_K494004 was constructed using <partinfo>BBa_K494001</partinfo> as a backbone and the cloning steps described before. Starting from <partinfo>BBa_K494003</partinfo>, a signal sequence was inserted downstream of the reporter protein mCherry. The main goal of our project is to construct and evaluate switchable elements based on terminators, our so called BioLOGICS in response of a short RNA signal strand. Therefore the signal needs to be incorporated in the plasmid. The inserted signal follows an IPTG inducible promoter, for tight regulation of the transcription. In theory every promoter can be used and any other DNA encoded signal or addition sequence can be cloned downstream of the reporter protein. Upon induction with IPTG the signal is transcribed and can in theory bind to the terminator stem loop, leading to antitermination and therefore detectable mCherry fluorescence. Another example of the same principle is shown in <partinfo>BBa_K494006</partinfo>. Again, it is no time is lost due to more than one cloning step, since like in the case of <partinfo>BBa_K494002</partinfo>, a negative control for measurements based on <partinfo>BBa_K494004</partinfo> is given by <partinfo>BBa_K494003</partinfo>. | |
| + | |||
{{:Team:TU Munich/Templates/ToggleBoxStart}} | {{:Team:TU Munich/Templates/ToggleBoxStart}} | ||
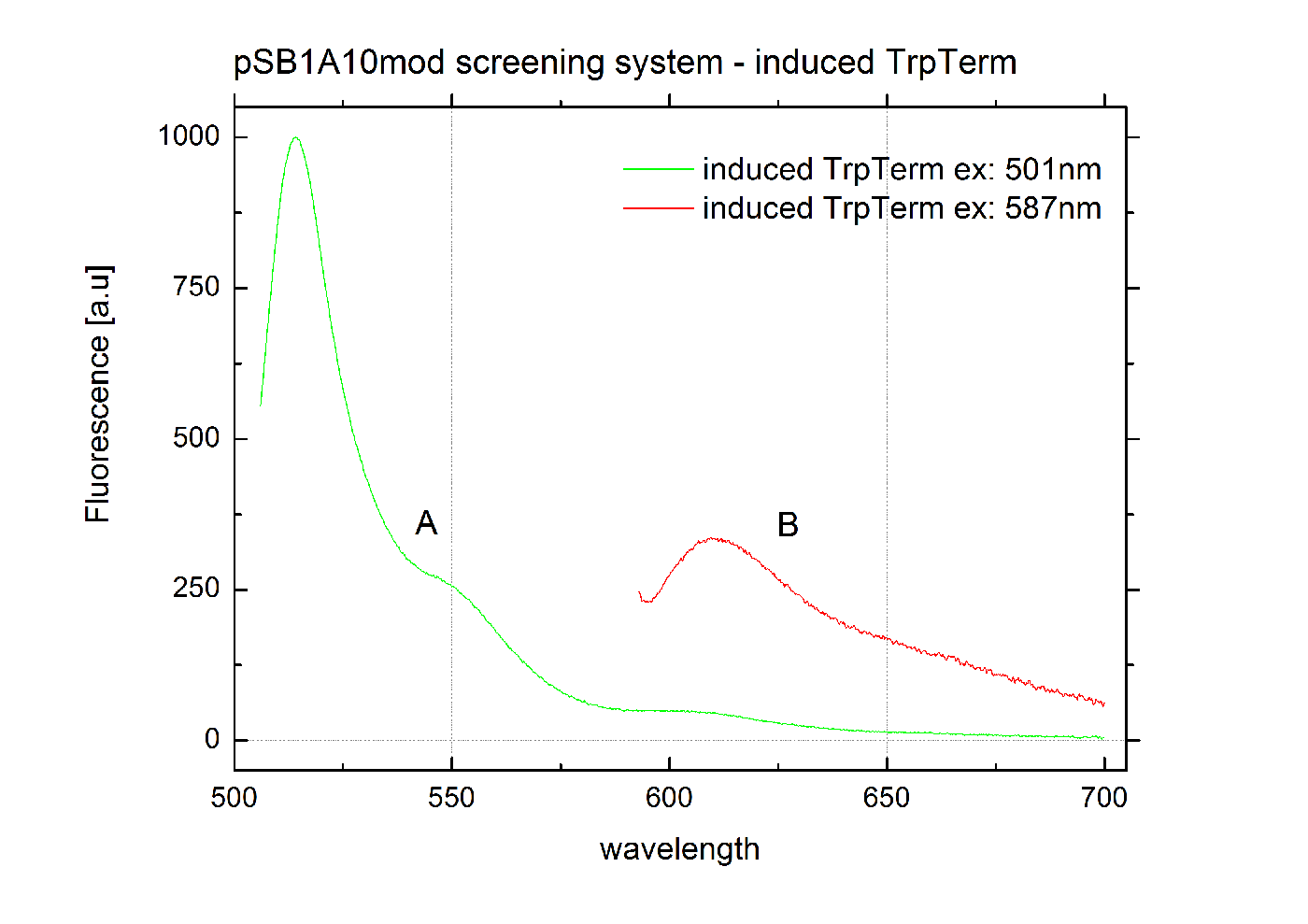
| - | [[Image:TUM2010_graphTrpTermInd.png|400px|thumb|center|Emission spectra of induced | + | [[Image:TUM2010_graphTrpTermInd.png|400px|thumb|center|Emission spectra of induced <partinfo>pSB1A10</partinfo>mod TrpTerm <partinfo>BBa_K494004</partinfo> ; A: eGFP fluorescence ex: 501 nm, B: mCherry fluorescence ex: 587 nm]] |
{{:Team:TU Munich/Templates/ToggleBoxEnd}} | {{:Team:TU Munich/Templates/ToggleBoxEnd}} | ||
| - | === Screening System: BBa_K494005 === | + | === Screening System: <partinfo>BBa_K494005</partinfo> === |
| + | BBa_K494005 is constructed following the same principles like <partinfo>BBa_K494003</partinfo>. Instead of the ''his-operon'' attenuation sequence, a Rho-independent terminator from ''E. coli'' which regulates the transcription of the ''trp-operon'' was chosen. The Trp-terminator does not inhibit transcription as efficiently as the His-terminator, mCherry fluorescence is still detectable in the measurements. <partinfo>BBa_K494005</partinfo> was used for further cloning of <partinfo>BBa_K494006</partinfo>. | ||
| + | {{:Team:TU Munich/Templates/ToggleBoxStart}} | ||
| + | [[Image:TUM2010_graphTrpTermInd.png|400px|thumb|center|Emission spectra of induced <partinfo>pSB1A10</partinfo>mod TrpTerm <partinfo>BBa_K494004</partinfo> ; A: eGFP fluorescence ex: 501 nm, B: mCherry fluorescence ex: 587 nm]] | ||
| + | {{:Team:TU Munich/Templates/ToggleBoxEnd}} | ||
| - | === Screening System: BBa_K494006 === | + | === Screening System: <partinfo>BBa_K494006</partinfo> === |
| + | BBa_K494006 again is similiar to <partinfo>BBa_K494004</partinfo>. Like in the case of <partinfo>BBa_K494004</partinfo>, a signal sequence which is constructed to bind into the hairpin-structure of the Trp-Terminator and therefore resolving it, is inserted downstream of the reporter protein. Since the Trp-terminator does not terminate as efficiently, the noise level is higher which must be concerned upon measurement evaluation. | ||
| + | {{:Team:TU Munich/Templates/ToggleBoxStart}} | ||
| + | [[Image:TUM2010_graphTrpTermInd.png|400px|thumb|center|Emission spectra of induced <partinfo>pSB1A10</partinfo>mod TrpTerm <partinfo>BBa_K494004</partinfo> ; A: eGFP fluorescence ex: 501 nm, B: mCherry fluorescence ex: 587 nm]] | ||
| + | {{:Team:TU Munich/Templates/ToggleBoxEnd}} | ||
| + | |||
| + | =Parts Falsification and Verification= | ||
| + | <br> | ||
| + | {| class="bordertable" width=700 align="center" style="text-align: center; font-size:80%" | ||
| + | |||
| + | |- align=”center” ß Textausrichtung in den Spalten | ||
| + | |||
| + | ! style="color:#0065bd;font-size:110%" | Identifier | ||
| + | |||
| + | ! style="color:#0065bd;font-size:110%" | Name | ||
| + | |||
| + | ! style="color:#0065bd;font-size:110%" | Part Type | ||
| + | |||
| + | ! style="color:#0065bd;font-size:110%" | Description | ||
| + | |||
| + | ! style="color:#0065bd;font-size:110%" | Validation | ||
| + | |||
| + | ! style="color:#0065bd;font-size:110%" | Availability | ||
| + | |||
| + | |- align=”center” | ||
| + | | <partinfo>BBa_J04450</partinfo> | ||
| + | | RFP Coding Device | ||
| + | | Reporter | ||
| + | | Basic BioBrick Insert for usage as cloning reporter | ||
| + | | style="background:#4e9d20"|worked | ||
| + | | existing | ||
| + | |||
| + | |- align=”center” | ||
| + | | <partinfo>BBa_R0011</partinfo> | ||
| + | | Promoter (lacI regulated, lambda pL hybrid) | ||
| + | | Regulatory | ||
| + | | IPTG inducable promoter | ||
| + | | style="background:#4e9d20"|worked | ||
| + | | existing | ||
| + | |||
| + | |- align=”center” | ||
| + | | <partinfo>BBa_I13453</partinfo> | ||
| + | | Pbad promoter | ||
| + | | Regulatory | ||
| + | | Arabinose inducable promoter | ||
| + | | style="background:#4e9d20"|worked | ||
| + | | existing | ||
| + | |||
| + | |- align=”center” | ||
| + | | <partinfo>BBa_J06702</partinfo> | ||
| + | | mCherry with RBS | ||
| + | | Reporter | ||
| + | | Fluorescent protein generator: mCherry | ||
| + | | style="background:#4e9d20"|worked | ||
| + | | existing | ||
| + | |||
| + | |- align=”center” | ||
| + | | <partinfo>pSB1A2</partinfo> | ||
| + | | pSB1A2 | ||
| + | | Plasmid Backbone | ||
| + | | Basic plasmid backbone with ampicillin resistance | ||
| + | | style="background:#4e9d20"|worked | ||
| + | | existing | ||
| + | |||
| + | |- align=”center” | ||
| + | | <partinfo>pSB1A3</partinfo> | ||
| + | | pSB1A3 | ||
| + | | Plasmid Backbone | ||
| + | | Basic plasmid backbone with ampicillin resistance | ||
| + | | style="background:#4e9d20"|worked | ||
| + | | existing | ||
| + | |||
| + | |- align=”center” | ||
| + | | <partinfo>pSB1K3</partinfo> | ||
| + | | High copy BioBrick assembly plasmid | ||
| + | | Plasmid Backbone | ||
| + | | Basic plasmid backbone with kanamycin resistance | ||
| + | | style="background:#4e9d20"|worked | ||
| + | | existing | ||
| + | |||
| + | |- align=”center” | ||
| + | | <partinfo>pSB1A10</partinfo> | ||
| + | | BioBrick backbone for measuring termination efficiency | ||
| + | | Plasmid Backbone | ||
| + | | Plasmid backbone with ampicillin resistance (based on pSB1A2) | ||
| + | | style="background:red"|failed<br>[[Team:TU_Munich/Parts#Falsification_of_pSB1A10 | more details]] | ||
| + | | existing | ||
| + | |} | ||
| + | <br> | ||
| - | + | ==Falsification of pSB1A10== | |
| - | ==pSB1A10== | + | |
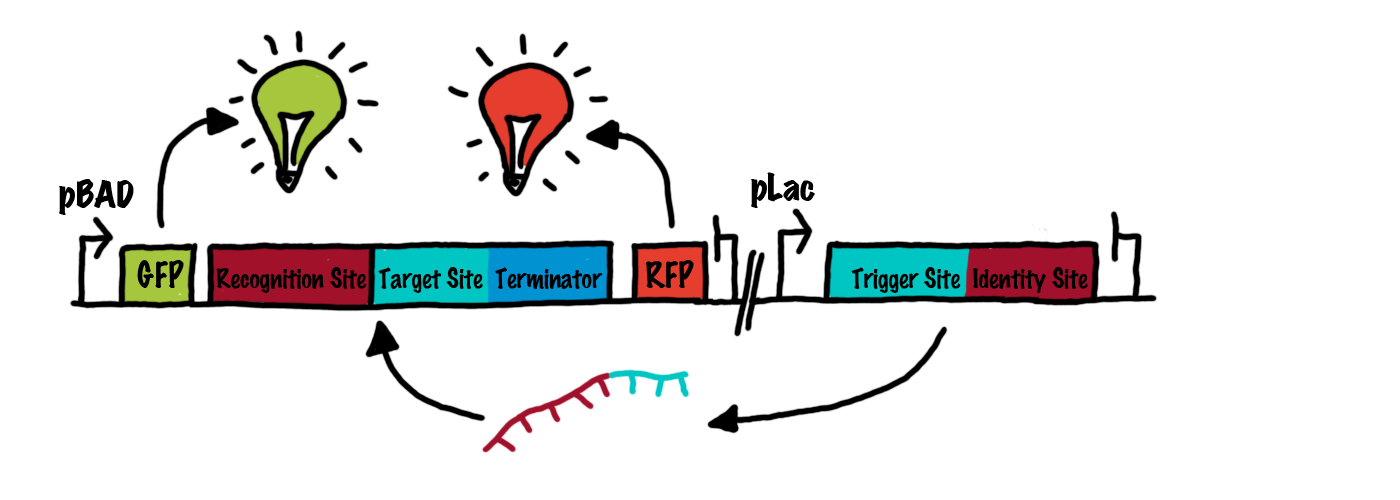
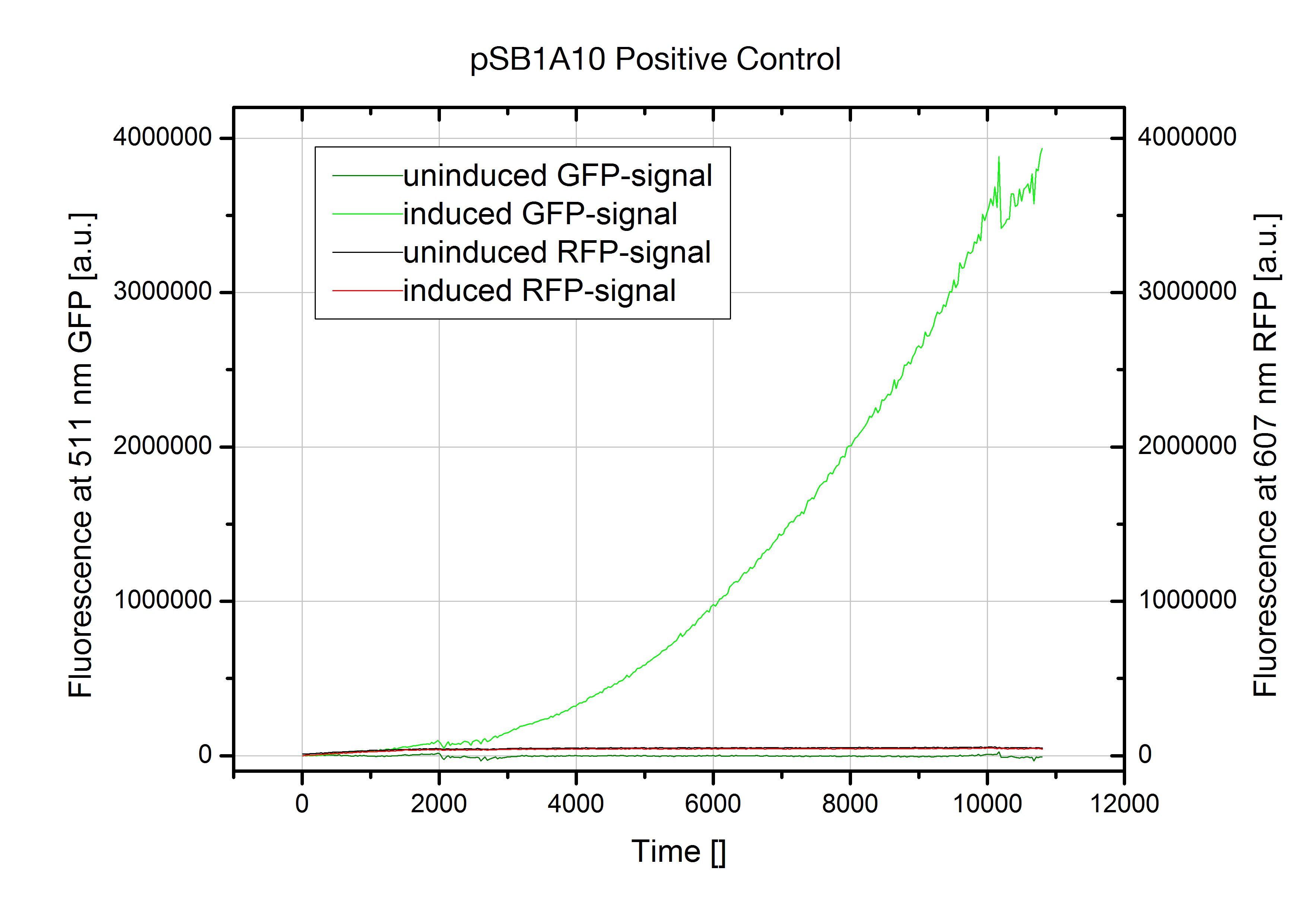
The Screening Plasmid pSB1A10 is intended for the characterization of an Input/Output for a PoPS based device on the basis of two fluorescent proteins. While the first fluorescent protein, eGFP quantifies the input from an arabinose induced promoter, the second reporter, RFP detects the output. | The Screening Plasmid pSB1A10 is intended for the characterization of an Input/Output for a PoPS based device on the basis of two fluorescent proteins. While the first fluorescent protein, eGFP quantifies the input from an arabinose induced promoter, the second reporter, RFP detects the output. | ||
| - | However, our experiments designed for testing | + | However, our experiments designed for testing switches revealed the part to be non-functional. In the current available form, RFP fluorescence is not induced even when the PoPS device does not interfere with the Polymerase. |
{{:Team:TU Munich/Templates/ToggleBoxStart}} | {{:Team:TU Munich/Templates/ToggleBoxStart}} | ||
{| | {| | ||
| Line 85: | Line 185: | ||
<html><a name="ref4"></a></html>[4] Stojanovic, M.N. and D.M. Kolpashchikov, Modular aptameric sensors. J. Am. Chem. Soc, 2004. 126(30): p. 9266-9270. | <html><a name="ref4"></a></html>[4] Stojanovic, M.N. and D.M. Kolpashchikov, Modular aptameric sensors. J. Am. Chem. Soc, 2004. 126(30): p. 9266-9270. | ||
| - | <html><a name=" | + | <html><a name="ref6"></a></html>[5] http://en.wikipedia.org/wiki/Logic_gate#Symbols |
| - | + | ||
<!-- ############## WIKI-PAGE STOPS HERE ############## --> | <!-- ############## WIKI-PAGE STOPS HERE ############## --> | ||
{{:Team:TU_Munich/Templates/End}} | {{:Team:TU_Munich/Templates/End}} | ||
Latest revision as of 19:37, 20 November 2010
|
|
Submitted Parts<groupparts align="center" width="100%">igem2010 TU_Munich</groupparts>
Malachite Green Binding Aptamer - <partinfo>BBa_K494000</partinfo>
The malachite green binding aptamer has been successfully used in screening systems being both robust and easy to produce. Aptamers provide specifities in the range of antibodies and can be evolved to target small molecules and proteins.[3] PlasmidsIn general we want to provide a new principle of gene regulation which can be further developed, tested and optimized by everybody. Therefore we focus on providing the parts needed for verification and testing of new individual switches. We provide a plasmid backbone which can be used for further cloning, a positive control to test the general functionality and measurements setups and the constructs we characterized for comparison. Screening system: Backbone <partinfo>BBa_K494001</partinfo>Backbone <partinfo>BBa_K494001</partinfo> is a new backbone which can be utilized not only for evaluation of terminator-based switches but also for testing basically all PoPS devices. In principle, <partinfo>BBa_K494001</partinfo> is a modified version of <partinfo>pSB1A10</partinfo>, which allows more flexibility in regard of the reporter protein and suits very well for testing and screening of switches based on bioLOGIC principles in vivo and with an in vitro translation assay. As an internal control, eGFP is integrated into the backbone to evaluate measurement setups and induction quality. eGFP is under the control of an pBAD promoter, therefore the whole pBAD promoter cassette being also located on <partinfo>BBa_K494001</partinfo>. In comparison to <partinfo>pSB1A10</partinfo> a new cloning site was created right after the eGFP protein which allows the insertion of every DNA encoded BioBrick reporter. Reporter proteins may be exchanged to fit the challenge and can be easily exchanged if not well-functioning.
Screening System: Positive Control <partinfo>BBa_K494002</partinfo>BBa_K494002 is the backbone <partinfo>BBa_K494001</partinfo> with an mCherry inserted into the standard BioBrick cloning site using EcoRI and PstI. mCherry was further equipped with an arabinose inducible promoter (pBAD) for good expression yield and can be used as a further reference and internal standard to compare measurements and setups. With the mCherry fluorescence in the red, a fast maturation time and a satisfying quantum yield, mCherry is the perfect complement to eGFP if fluroscence is used as an output. mCherry is expressed together with eGFP after induction with arabinose without delaying elements in between both proteins.
Here we present a ready to use composite part consisting of our screening system <partinfo>pSB1A10</partinfo>mod <partinfo>BBa_K494001</partinfo>, the PBad Promotor <partinfo>BBa_I13453</partinfo> and the reporter protein mCherry <partinfo>BBa_J06702</partinfo> including RBS and double Terminator <partinfo>BBa_B0015</partinfo>. This part belongs to the screening system and is intended as positive control. Close Screening System: <partinfo>BBa_K494003</partinfo> - exemplary insert into <partinfo>BBa_K494001</partinfo>With the reporter protein inserted, EcoRI and XhoI upstream of the chosen reporter protein and SpeI and PstI downstream the reporter protein are now free for insertion of further proteins. In the case of <partinfo>BBa_K494003</partinfo>, <partinfo>BBa_K494001</partinfo> was utilized to evaluate a terminator for both termination efficiency and evaluation of antitermination. To test the termination efficiency, a terminator was inserted upstream of the reporter protein mCherry, in this case a Rho-independent terminator based on the regulative hairpin in front of the his-operon from Salmonella enterica. With this strong terminator right before mCherry, no mCherry fluorescence can be detected anymore while the eGFP fluorescences grows steadily over time. Another example of the same principle is shown in <partinfo>BBa_K494005</partinfo>. A further part can be inserted following the reporter protein using SpeI and PstI, this was done in the case of <partinfo>BBa_K494004</partinfo>.
Here we present a ready to use composite part consisting of our screening system <partinfo>pSB1A10</partinfo>mod <partinfo>BBa_K494001</partinfo>, the PBad Promotor BBa_I13453, the His Terminator and the reporter protein mCherry BBa_J06702 including RBS and double Terminator BBa_B0015. This part belongs to the screening system and is intended as negative control.
Close
Screening System: <partinfo>BBa_K494004</partinfo> - exemplary insert into <partinfo>BBa_K494001</partinfo>BBa_K494004 was constructed using <partinfo>BBa_K494001</partinfo> as a backbone and the cloning steps described before. Starting from <partinfo>BBa_K494003</partinfo>, a signal sequence was inserted downstream of the reporter protein mCherry. The main goal of our project is to construct and evaluate switchable elements based on terminators, our so called BioLOGICS in response of a short RNA signal strand. Therefore the signal needs to be incorporated in the plasmid. The inserted signal follows an IPTG inducible promoter, for tight regulation of the transcription. In theory every promoter can be used and any other DNA encoded signal or addition sequence can be cloned downstream of the reporter protein. Upon induction with IPTG the signal is transcribed and can in theory bind to the terminator stem loop, leading to antitermination and therefore detectable mCherry fluorescence. Another example of the same principle is shown in <partinfo>BBa_K494006</partinfo>. Again, it is no time is lost due to more than one cloning step, since like in the case of <partinfo>BBa_K494002</partinfo>, a negative control for measurements based on <partinfo>BBa_K494004</partinfo> is given by <partinfo>BBa_K494003</partinfo>.
Screening System: <partinfo>BBa_K494005</partinfo>BBa_K494005 is constructed following the same principles like <partinfo>BBa_K494003</partinfo>. Instead of the his-operon attenuation sequence, a Rho-independent terminator from E. coli which regulates the transcription of the trp-operon was chosen. The Trp-terminator does not inhibit transcription as efficiently as the His-terminator, mCherry fluorescence is still detectable in the measurements. <partinfo>BBa_K494005</partinfo> was used for further cloning of <partinfo>BBa_K494006</partinfo>. Screening System: <partinfo>BBa_K494006</partinfo>BBa_K494006 again is similiar to <partinfo>BBa_K494004</partinfo>. Like in the case of <partinfo>BBa_K494004</partinfo>, a signal sequence which is constructed to bind into the hairpin-structure of the Trp-Terminator and therefore resolving it, is inserted downstream of the reporter protein. Since the Trp-terminator does not terminate as efficiently, the noise level is higher which must be concerned upon measurement evaluation. Parts Falsification and Verification
Falsification of pSB1A10The Screening Plasmid pSB1A10 is intended for the characterization of an Input/Output for a PoPS based device on the basis of two fluorescent proteins. While the first fluorescent protein, eGFP quantifies the input from an arabinose induced promoter, the second reporter, RFP detects the output. However, our experiments designed for testing switches revealed the part to be non-functional. In the current available form, RFP fluorescence is not induced even when the PoPS device does not interfere with the Polymerase.
Although induction with arabinose worked fine indicated by GFP fluorescence, we could not detect any RFP output. The kinetic measurment on the left shows pSB1A10 postive control with a nonsense sequence inserted into the BioBrick site between the two fluorescent proteins GFP and RFP. Nevertheless induction does not trigger any RFP expression. Therefore no output signal is created in our experimantal setup. This fact renders the part as it is unsuitable for testing PoPS-based devices. We first assumed degradation of RFP-mRNA which is known to have an RNase site might be responsible. But comparison with our new screening system reveals a transcriptional problem to be most likely as replacing RFP did not improve the construct. Though, introducing a second arabinose promoter finally resulted in the desired output signal.
References[1] Grate, D. and C. Wilson, Laser-mediated, site-specific inactivation of RNA transcripts. Proceedings of the National Academy of Sciences of the United States of America, 1999. 96(11): p. 6131. [2] Babendure, J.R., S.R. Adams, and R.Y. Tsien, Aptamers switch on fluorescence of triphenylmethane dyes. J. Am. Chem. Soc, 2003. 125(48): p. 14716-14717. [3] Rowe, W., M. Platt, and P.J.R. Day, Advances and perspectives in aptamer arrays. Integrative Biology, 2009. 1(1): p. 53-58. [4] Stojanovic, M.N. and D.M. Kolpashchikov, Modular aptameric sensors. J. Am. Chem. Soc, 2004. 126(30): p. 9266-9270. [5] http://en.wikipedia.org/wiki/Logic_gate#Symbols |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"