Team:Groningen/23 August 2010
From 2010.igem.org
| (16 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Team:Groningen/Header}} | {{Team:Groningen/Header}} | ||
| - | + | '''Week 34''' | |
| + | ---- | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
'''Exression experiment''' | '''Exression experiment''' | ||
| Line 16: | Line 10: | ||
'''Peter & David''' | '''Peter & David''' | ||
| - | |||
<br> | <br> | ||
For this experiment, the following B. subtilis 168 strains were used: | For this experiment, the following B. subtilis 168 strains were used: | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
<br> | <br> | ||
| Line 77: | Line 63: | ||
<br> | <br> | ||
| - | [[Image: | + | [[Image:GrowthCurveGR2.jpg|400px]] |
<br> | <br> | ||
| Line 89: | Line 75: | ||
<br> | <br> | ||
| - | [[Image: | + | [[Image:THTGR2.jpg|400px]] |
<br> | <br> | ||
| Line 100: | Line 86: | ||
<br> | <br> | ||
| - | [[Image:SDS- | + | [[Image:SDS-PAGEGR2.jpg|400px]] |
<br> | <br> | ||
| Line 114: | Line 100: | ||
''Bacillus Subtilis'' cultures were grown overnight in a Petridish containing 20mL of LB medium and a piece of ceramics to spurr the biofilm formation. The following cultures wehre grown: | ''Bacillus Subtilis'' cultures were grown overnight in a Petridish containing 20mL of LB medium and a piece of ceramics to spurr the biofilm formation. The following cultures wehre grown: | ||
'' | '' | ||
| - | Bacillus Subtilis'', ''Bacillus Subtilis'' ΔTasA, ''Bacillus Subtilis'' | + | Bacillus Subtilis'', ''Bacillus Subtilis'' ΔTasA, ''Bacillus Subtilis'' with chaplin E1, ''Bacillus Subtilis'' with chaplin H1, ''Bacillus Subtilis'' ΔTasA with chaplin E1, ''Bacillus Subtilis'' ΔTasA with chaplin H1 |
All cultures where inoculated with 100uL of overnight culture. All cultures where run in duplicate, one induced with 1%subtilin, and one without subtilin as a negative control. | All cultures where inoculated with 100uL of overnight culture. All cultures where run in duplicate, one induced with 1%subtilin, and one without subtilin as a negative control. | ||
| Line 131: | Line 117: | ||
Our assumption is that either: the expression of chaplins has no visible effect on biofilm formation or, that the subtilin was degraded over night and no proper expression of chaplins took place. | Our assumption is that either: the expression of chaplins has no visible effect on biofilm formation or, that the subtilin was degraded over night and no proper expression of chaplins took place. | ||
| - | '''Modellers:''' Read articles, think about model etc. ''Joël, Laura, Djoke'' | + | '''Geeske''' |
| + | |||
| + | Started to make the following constructs: | ||
| + | |||
| + | -pNZ8901_EH (combination) | ||
| + | |||
| + | -pNZ8901_C | ||
| + | |||
| + | -pNZ8901_dC (adapted in signal sequence) | ||
| + | |||
| + | -pNZ8901_CS (Chaplin C and Sortase) | ||
| + | |||
| + | -pNZ8901_dCS | ||
| + | |||
| + | -pNZ8901_CH | ||
| + | |||
| + | |||
| + | However halted the attempts to check the ''B. sub'' NZ8900 pNZ8901_E, ''B. sub'' NZ8900 pNZ8901_H clones and ΔTasA versions with mini-prep and gelelectroforese for presence of the pNZ8901-plasmids. Only ''B. sub'' ΔTasA NZ8900 pNZ8901_E (sometimes referred to as E1) showed plasmid. This could explain all the expression results. There was no explanation, however, for why the strains did grow on the selective antibiotics. | ||
| + | |||
| + | |||
| + | '''Modellers:''' | ||
| + | Read articles, think about model etc. ''Joël, Laura, Djoke'' | ||
| + | |||
| + | Still researching information required for information standard ''Arend'' | ||
| + | |||
| + | Working on killswitch model, find information about various killswitch systems. ''Laura'' | ||
| + | |||
| + | |||
| + | '''Safety''' | ||
| + | |||
| + | Talked to Juke Lolkema about safety issues, wrote the part on safety for the wiki. ''Djoke and Laura'' | ||
| + | |||
| + | '''Jorrit''' | ||
| + | |||
| + | Repeated transformation of week 33 of the biofilm forming strains, again no colonies visible | ||
| + | helped David & Peter processing samples | ||
| + | Cut CCH1, dCS and pNZ plasmid with EcoR1 FD, Spe1 FD, checked on gel | ||
| + | isolated product from gel with Bioké kit and nanodropped. | ||
| + | |||
| + | |||
| + | '''Arend Jan''' | ||
| + | |||
| + | |||
| + | Two variants of the srfA promoter will be made into biobrick parts by amplifying them from the genome and flanking them with the prefix and suffix. PCR Primers were desinged and a PCR was performed using varying conditions. | ||
| + | |||
| + | |||
| + | [[Image:25-08-10gn.jpg|300px|thumb|left|srfA promoter PCR with varying template dilutions and DMSO addition]] | ||
| + | <br style="clear: both" /> | ||
| + | The products were purified and ligated into the pSB1C3 backbone, both with and without the BBa_E0240 GFP part. Transformation plates were grown over the weekend. | ||
{{Team:Groningen/Footer}} | {{Team:Groningen/Footer}} | ||
Latest revision as of 03:44, 28 October 2010
Week 34
Exression experiment
Peter & David
For this experiment, the following B. subtilis 168 strains were used:
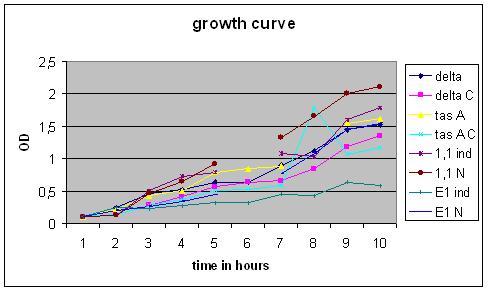
All cultures were grown overnight at 37 degrees Celsius in a shaker room, the appropriate antibiotics were used at all points in time during this experiment.
Overnight cultures were used to dilute to a B. subtilis culture of 0,1 OD, these strains were divided into ‘’induced’’ and ‘’non-induced’’. Induction with 0,5% subtilin was done at a OD of 0,5 (approximately 2,5 hours after growth of the 0,1 culture started).
After that the OD of the cultures was measured every .. hours.
Sample preperation
After .. hours, .. after induction, the samples were collected and processed. The following procedures were used:
Pellet preperation (PelletPrepGR)
Supernatant processing (SupernatantPrepGR)
Cell disruption (ExtractionCellWallsGR)
Lysozyme preperation (LysozymePrepGR)
Analysis was done using SDS-PAGE (SDS-PAGEGR) and THT staining (THTstainingGR).
Results:
Growth Curve
THT Staining
SDS-PAGE
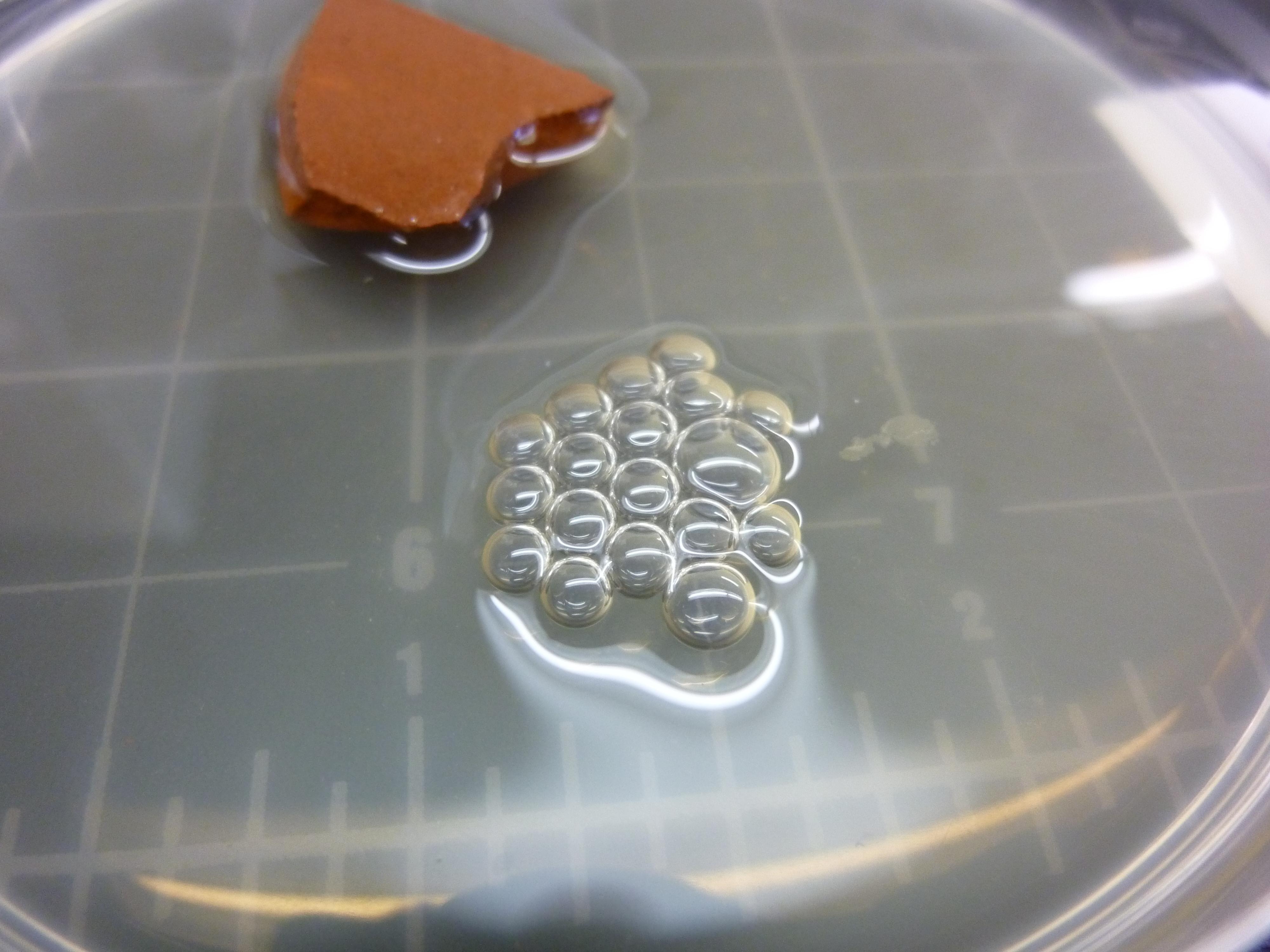
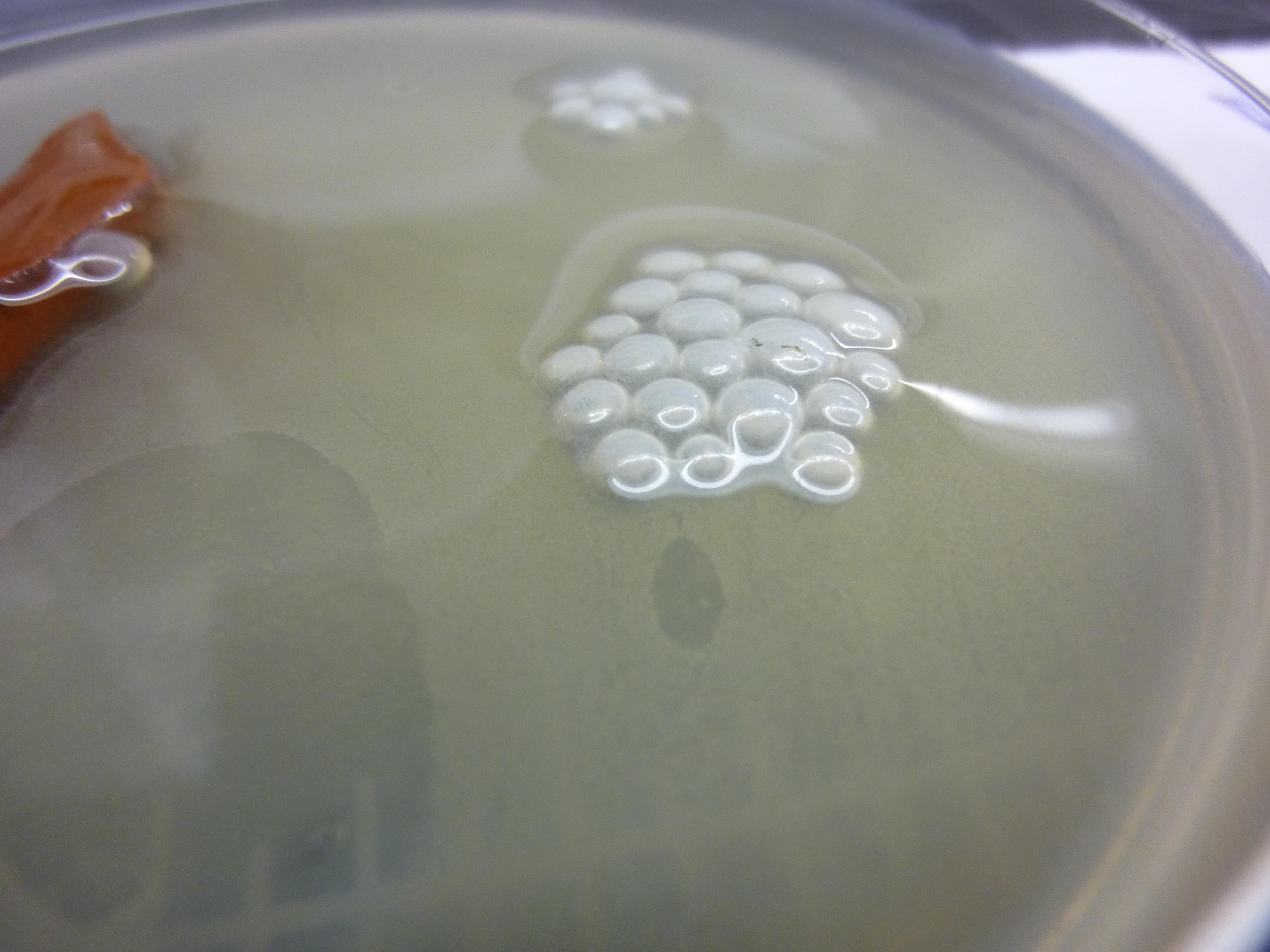
Biofilm formation experiment
Bacillus Subtilis cultures were grown overnight in a Petridish containing 20mL of LB medium and a piece of ceramics to spurr the biofilm formation. The following cultures wehre grown: Bacillus Subtilis, Bacillus Subtilis ΔTasA, Bacillus Subtilis with chaplin E1, Bacillus Subtilis with chaplin H1, Bacillus Subtilis ΔTasA with chaplin E1, Bacillus Subtilis ΔTasA with chaplin H1
All cultures where inoculated with 100uL of overnight culture. All cultures where run in duplicate, one induced with 1%subtilin, and one without subtilin as a negative control.
We assumed that the ΔTasA would not form a biofilm and the normal phenotype could be restored by the expression of chaplins. Furthermore we assumed that the normal Bacillus Subtilis biofilm would have atered properties.
No such effects could be observed all ΔTasA cultures showed the same non biofilm phenotype
The same goes for all Bacillus Subtilis cultures with TasA, the were all not affected by induction of Chaplins, and showed a normal biofiml forming phenotype.
Our assumption is that either: the expression of chaplins has no visible effect on biofilm formation or, that the subtilin was degraded over night and no proper expression of chaplins took place.
Geeske
Started to make the following constructs:
-pNZ8901_EH (combination)
-pNZ8901_C
-pNZ8901_dC (adapted in signal sequence)
-pNZ8901_CS (Chaplin C and Sortase)
-pNZ8901_dCS
-pNZ8901_CH
However halted the attempts to check the B. sub NZ8900 pNZ8901_E, B. sub NZ8900 pNZ8901_H clones and ΔTasA versions with mini-prep and gelelectroforese for presence of the pNZ8901-plasmids. Only B. sub ΔTasA NZ8900 pNZ8901_E (sometimes referred to as E1) showed plasmid. This could explain all the expression results. There was no explanation, however, for why the strains did grow on the selective antibiotics.
Modellers:
Read articles, think about model etc. Joël, Laura, Djoke
Still researching information required for information standard Arend
Working on killswitch model, find information about various killswitch systems. Laura
Safety
Talked to Juke Lolkema about safety issues, wrote the part on safety for the wiki. Djoke and Laura
Jorrit
Repeated transformation of week 33 of the biofilm forming strains, again no colonies visible helped David & Peter processing samples Cut CCH1, dCS and pNZ plasmid with EcoR1 FD, Spe1 FD, checked on gel isolated product from gel with Bioké kit and nanodropped.
Arend Jan
Two variants of the srfA promoter will be made into biobrick parts by amplifying them from the genome and flanking them with the prefix and suffix. PCR Primers were desinged and a PCR was performed using varying conditions.
The products were purified and ligated into the pSB1C3 backbone, both with and without the BBa_E0240 GFP part. Transformation plates were grown over the weekend.
 "
"