Team:Imperial College London/Human Practices/Report
From 2010.igem.org
m |
|||
| (20 intermediate revisions not shown) | |||
| Line 4: | Line 4: | ||
|style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Human Practices Report | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Human Practices Report | ||
|- | |- | ||
| - | | | + | |Our project focuses on the design of a system that gives a rapid response, because we noticed that many previous biosensor projects had only achieved relatively slow responses to a stimulus. Our concept is an adaptable, modular and robust fast response system, which is fundamental for modern applications in synthetic biology. |
One possible application for this fast response module is in the detection of water-borne parasites. We realised that synthetic biology is rarely used in the fight against neglected tropical diseases (NTDs), and we wanted to be pioneers in this field. | One possible application for this fast response module is in the detection of water-borne parasites. We realised that synthetic biology is rarely used in the fight against neglected tropical diseases (NTDs), and we wanted to be pioneers in this field. | ||
| - | Over 1 billion people - a sixth of the world’s population - suffer from one or more | + | Over 1 billion people - a sixth of the world’s population - suffer from one or more NTDs (WHO, 2007). Despite affecting over 90% of the world’s population in one way or another, only 10% of worldwide health research funding goes into finding preventative or curative treatment for NTDs. This statistic was published by the Global Health Forum and is now referred to as the ‘10/90 gap’ (Global Forum for Health Research, 1999). The 10/90 gap probably exists because of the fact that the majority of people affected by NTDs live in developing countries (Hotez ''et al'', 2006), and so profit margins for NTD drugs are negligible. Therefore, Western pharmaceutical companies are unlikely to see countries in which NTDs are endemic as high priority commercial markets (Hotez ''et al'', 2006). |
The World Health Organisation lists the following diseases as the main NTDs (WHO, 2007): | The World Health Organisation lists the following diseases as the main NTDs (WHO, 2007): | ||
| Line 15: | Line 15: | ||
* Dengue/dengue haemorrhaegic fever | * Dengue/dengue haemorrhaegic fever | ||
* Dracunculiasis (guinea-worm disease) | * Dracunculiasis (guinea-worm disease) | ||
| - | * Endemic treponematoses | + | * Endemic treponematoses |
* Human African trypanosomiasis (sleeping sickness) | * Human African trypanosomiasis (sleeping sickness) | ||
* Leishmaniasis | * Leishmaniasis | ||
| Line 26: | Line 26: | ||
| - | + | '''Here's an interview with Professor Alan Fenwick on the subject of NTDs:''' | |
| - | + | <html><object width="571" height="366"><param name="movie" value="http://www.rockhopper.tv/flash/mxmlVideoPlayer.swf?id=207&src=http://www.rockhopper.tv/webservices/get-programme2.aspx&site=rockhopper"></param><param name="allowFullScreen" value="true"></param><param name="allowscriptaccess" value="always"></param><embed src="http://www.rockhopper.tv/flash/mxmlVideoPlayer.swf?id=207&src=http://www.rockhopper.tv/webservices/get-programme2.aspx&site=rockhopper" type="application/x-shockwave-flash" allowscriptaccess="always" allowfullscreen="true" width="571" height="366"></embed></object></html> | |
| - | |||
| - | In | + | NTDs are most commonly found in remote, rural areas of sub-Saharan Africa, Asia and Latin America (Hotez ''et al'', 2006). The effects of NTDs are wide-ranging and include long-term disability, disfigurement, impaired childhood growth, adverse pregnancy outcomes and reduced economic productivity (WHO, 2003). In fact, the effect on worker productivity causes billions of dollars to be lost each year, and retains a country in a state of poverty (Hotez ''et al'', 2006; Molyneux et al, 2005). Added to these problems is that of climate change, which is creating favourable conditions for the spread of many infectious diseases and their vectors, and so it is expected that in the years to come their effects will be felt all the more gravely (Patz ''et al'', 1996). |
| - | + | |} | |
| + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Schistosomiasis | ||
| + | |- | ||
| + | |Schistosomiasis, also known as bilharzia, is an NTD that affects over 200 million people worldwide (Steinmann ''et al'', 2005) and has drastic effects on the economic viability of the country in which it is endemic (Sturrock, R. F., 1993). The ''Schistosoma'' species are human parasites found in sub-Saharan Africa, Latin America and South-East Asia (Liu ''et al'', 2010). The parasite has a complicated lifecycle, involving two hosts; freshwater snails and humans, and infects humans by breaking through the skin during contact with contaminated water (Gryseels ''et al'', 2006; Coon ''et al'', 2005). | ||
| - | The | + | The construction of man-made water systems has extended snail habitats by creating favourable conditions for their survival and reproduction. As people move closer to lakes, dams and other irrigations systems an optimal environment for the transmission of the parasite results, thus making schistosomiasis even more of a major global health problem (Fenwick, 2009). |
| - | + | In recent years, the treatment of schistosomiasis, has improved markedly due to various schemes of chemotherapy, including the administration of praziquantel (Fenwick, 2009). This drug is effective as a single dose, can be administered by primary healthcare teams and directly affects the ''Schistosoma'' parasite (Coon, 2005). However, it should be noted that the primary aim of these schemes is to reduce morbidity rather than prevent transmission. | |
| - | + | At present, most of the detection of ''Schistosoma'' is only possible after it has infected the human host. There are two main types of diagnosis; antibody detection in blood serum, or egg detection in urine and stool samples (Liu ''et al'', 2010). The latter is more commonly used but it is a lengthy and uneconomical technique. The detection of ''Schistosoma'' in the local environment is done rarely, as it is time-consuming and can require cumbersome equipment. Another drawback is the low sensitivity of the tests. | |
| + | The infective form of ''Schistosoma'', called a cercaria, secretes proteases upon detection of human skin lipids (McKerrow & Salter, 2002). These proteases enable the cercariae to break through the skin barrier and infect the host. We intend to use these proteases to detect the cercariae, because they can trigger a synthetic signaling pathway in our bacterium. | ||
| - | + | We have discussed a wide range of ethical, legal and social implications (ELSIs) of our project in a [https://2010.igem.org/Team:Imperial_College_London/Human_Practices/Workshop Human Practices Workshop], an [https://2010.igem.org/Team:Imperial_College_London/Human_Practices/Panel Interdisciplinary Human Practices Panel Discussion] and with [[https://2010.igem.org/Team:Imperial_College_London/Human_Practices/Meetings various experts] in the field. This has allowed us to ensure that our design specifcaitons are influenced by Human Practices. | |
| - | |||
| + | |} | ||
| + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Misuse | ||
| + | |- | ||
| + | |As with any scientific development, there is always the potential for misuse. We have debated who we think should have access to methods used in synthetic biology and in particular, our product. However, using synthetic biology in its current state requires huge amounts of expertise which is only possessed by research institutions and some commercial companies. Nevertheless, it has been proposed that some military organisations may possess sufficient resources to be capable of manipulating some developments for potentially detrimental ends, which might be one reason why funding for biodefence research has rocketed since 9/11 (Schmidt, 2008). | ||
| - | '' | + | As synthetic biology develops as a field, the ease with which systems are put together and the level of automation will improve, and thus entry barriers will diminish. Technology will become more affordable, especially as there is an ‘open source’ ethos within the field of synthetic biology (this will be further discussed in ‘Legal Factors’ and 'Political Factors and Regulation', where we propose an independent, international governing body for synthetic biology). |
| + | |} | ||
| + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Biosafety and Environmental Risks | ||
| + | |- | ||
| + | |Of particular concern is the uncontrolled release of Genetically Modified Organisms (GMOs), either intentional or accidental, into the environment. The prospect of contaminating groundwater is particularly alarming, especially in locations where coordination of water treatment is under-developed, as might be the case in some developing countries. We have considered how uncontrolled release would affect the local environment, and the biodiversity of its ecosystem. This led us to opt for a chassis that was already endemic in most environments. Our choice of chassis was ''B. subtilis'', which is a bacterium that is often found in soil and is non-pathogenic. | ||
| - | + | For more information, please see our '''[https://2010.igem.org/Team:Imperial_College_London/Safety Safety Page]'''. | |
| - | |||
| - | + | '''Integration into the ''B. subtilis'' genome disrupts essential genes''' | |
| - | + | To make the detection module we will integrate the required genes as two separate pieces of DNA (cassettes) directly into the ''B. subtilis'' genome. In order to limit the lifetime of the bacterium in the environment, we will integrate the cassettes at areas in the genome such that they will interfere with essential genes. One of these essential genes is needed to metabolise complex sugars (amyE) and the other is required for pyrimidine biosynthesis (pyrD), which are required for DNA synthesis. Additionally, all lab strains of ''B. subtilis'' are tryptophan knock-outs. As a result, the final version of our product would only be able to survive in special conditions where these additional nutrients are provided. Therefore, if it were to be released into the environment, it would, in theory, only survive for a limited period of time (this would be tested experimentally to quantify the exact lifetime of the bacterium in different environments and conditions). | |
| - | + | These [https://2010.igem.org/Team:Imperial_College_London/Chassis vectors] are now on the Registry and available for use. | |
| - | + | ||
| + | '''Removal of antibiotic genes using Dif excision''' | ||
| - | + | Most techniques of introducing new genes into organisms require additional selection genes; these usually confer resistance to a particular antibiotic such as spectinomycin. Our design will also include these genes, however additional Dif sequences built into our design will ensure that these genes are quickly removed from the bacterium, using an inherent control mechanism. As a result, the bacteria used in our final product will not be antibiotic resistant. Integration directly into the genome also ensures that the detection module genes will remain in the organism long enough for the kit to be used effectively. | |
| - | |||
| - | + | '''The product of the output reaction leads to cell death''' | |
| + | An additional level of confidence is that a component of the reaction mixture, called [https://2010.igem.org/Team:Imperial_College_London/Modules/Fast_Response catechol], actually kills the bacteria a few hours after it is added. We have tested this and results can be found in [https://2010.igem.org/Team:Imperial_College_London/Results/Exp4 Results]. | ||
| - | + | Nevertheless, we are aware that in order for this to occur, the catechol must be added appropriately, and this relies on the correct use of the detection kit. However, if the bacteria were released into the environment without being exposed to catechol, the original genome disruption would be sufficient to ensure that the bacteria would not survive. | |
| - | |||
| - | + | We recognise that biological systems are inherently unpredictable and have the capacity to change. Nevertheless, we believe that the benefits of controlling the population and spread of ''Schistosoma'' makes this an important project to pursue on the proviso that biosafety issues and environmental impacts are closely monitored, controlled and managed. | |
| + | We advocate continued, regular testing of the bacteria in order to ensure that any mutations, which would result in the deviation from its function, are noticed early and so the problem can be remediated. We would ensure that there are thorough, controlled and longitudinal studies of the effects of the bacteria on a range of environments and seasons, prior to the marketing of the product. | ||
| + | |} | ||
| + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Political Factors and Regulation | ||
| + | |- | ||
| + | |Regulation of the use of synthetic biology is a huge concern of both synthetic biologists and the wider public. We have debated whether regulation should be external or internal, and government-led or –funded. Self-regulation could be an effective way of ensuring that rules are adhered to, but with a lack of external scrutiny, it is unlikely to be sufficient for the public to feel entirely confident about the issues raised. This is due to the difficulty in keeping track of such a diffuse network of researchers (Schmidt, 2008) and the fact that there is always the potential for people to ignore the rules. We therefore believe that an international, independent body should monitor organisations working in the field of synthetic biology, and that there should be transparency in all their dealings. | ||
| - | + | We wanted to know what control measures were in place at our university and whether or not the government plays a role in this. At Imperial College London, the GM safety committee is responsible for ensuring that all research adheres to the regulations set down by the Health and Safety Executive (HSE). The first stage in the commencement of a project is the completion of a comprehensive risk assessment, which is submitted to the GM safety committee, which will then (i) approve the risk assessment and (ii) identify control measures. These control measures then need to be put in place. If the GM classification is Class 2 or higher, the project must be discussed with the Director of Occupational Health to determine whether health surveillance is necessary. | |
| - | + | |} | |
| + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Biosafety and Environmental Risks | ||
| + | |- | ||
| + | |A legal framework to control the use and ownership of the developments of synthetic biology is essential in order to establish the open source philosophy. We are aware that copyrights and patents can limit the scope of further research, because information is not accessible or research centres must pay heavily to use such innovations. There are many arguments for the implementation of open source synthetic biology, as opposed to using patenting laws. It has been suggested that open source projects drive innovation within the field, but they can also present huge logistical problems in terms of regulation upon diffusion of the technology (Schmidt, 2008). | ||
| - | + | There has been extensive media coverage concerning access to medicines and technology in the fight against NTDs. In the majority of cases, strong intellectual property rights (IPR) regulations rule out the generic production of drugs so that no alternatives to costly medicines are available. The majority of NTDs occur in developing countries, where healthcare provision is often poor and there are few resources available in terms of NTD medication. Strong IPR regulations for NTD drugs are often seen as unnecessary because pharmaceutical companies will not generate profits because people who can afford the drugs do not need them, and vice versa. Wider access to NTD drugs could be achieved by ensuring that research is available through open source. | |
| - | |||
| + | |} | ||
| + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Economic factors | ||
| + | |- | ||
| + | |We have ensured that the cost of production of the detection kit is low enough to make it affordable to the people who need it. This was achieved by designing a product that is economical to manufacture and uses inexpensive chemicals in the process. | ||
| - | + | The role of industry in synthetic biology is a controversial one. Industry is driven by profit, and we would like to ensure that no company has a monopoly in any particular area through the ownership of one or more of our modules. Ideally, we would like the technology to be available for the manufacturing of generic products. | |
| - | It is very important to us that the communities who would benefit from the detection kits have access to them and so they must be affordable and available in appropriate locations. Accessibility to the detection kits would depend on the following factors: | + | In particular, universities are centres of learning, and are not profit-generating businesses. We therefore believe that it is essential that research done at universities is accessible and can be used to benefit others. This could be achieved by making the modules available through open source, or by licensing the modules to different companies so that no one company has a monopoly. |
| + | |||
| + | |} | ||
| + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Social factors | ||
| + | |- | ||
| + | |It is very important to us that the communities who would benefit from the detection kits have access to them and so they must be affordable and available in appropriate locations. Accessibility to the detection kits would depend on the following factors: | ||
*Affordability | *Affordability | ||
*Distribution & ease of transportation | *Distribution & ease of transportation | ||
| Line 97: | Line 128: | ||
We also considered the type of organisation who would distribute the detection kits. This could be done by government organisations and local authorities, which would be an ideal solution because it would allow developing countries to organise and manage their local distribution and use of the kits, or use existing infrastructure if it is already in place. However, this may not be the best option if government resources are poor or if there is a threat of corruption or discrimination. If this were the case, aid programs and NGOs may be in a better position to distribute the kits. | We also considered the type of organisation who would distribute the detection kits. This could be done by government organisations and local authorities, which would be an ideal solution because it would allow developing countries to organise and manage their local distribution and use of the kits, or use existing infrastructure if it is already in place. However, this may not be the best option if government resources are poor or if there is a threat of corruption or discrimination. If this were the case, aid programs and NGOs may be in a better position to distribute the kits. | ||
| - | We do not believe that our detection kit on its own can solve the problem of | + | We do not believe that our detection kit on its own can solve the problem of ''Schistosoma''. Improved sanitation and education is needed in combination with the environmental control of parasites. It would be necessary to carry out an assessment of the level of education regarding parasitic infections, in particular schistosomiasis, in order to determine where further education was needed. This education programme could be given alongside the training in the use of the detection kit to ensure the long-term feasibility of our project, and also to overcome any cultural boundaries there may be. |
It is also essential that we determine the social acceptability of the kits before we finalise the design. During this process, it is possible that we would find that different designs are more appropriate in different settings, and the overall design may be needed to be tailored to a specific community, depending on the setting in which it is implemented. | It is also essential that we determine the social acceptability of the kits before we finalise the design. During this process, it is possible that we would find that different designs are more appropriate in different settings, and the overall design may be needed to be tailored to a specific community, depending on the setting in which it is implemented. | ||
| Line 104: | Line 135: | ||
| - | + | |} | |
| + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Design Specifications | ||
| + | |- | ||
| + | |Due to the constraints imposed by the environment in which most NTDs occur, our detection kit needs to be portable to resource-poor settings, affordable, easy to use and store, and not dangerous if released into the environment. | ||
| - | + | We considered the benefits of targeting the detection kit for use by organisations instead of domestic use. One advantage is that training in the use of the kit would only need to be given to employees of the organisations, and not to whole communities, thus making the system much more viable in terms of cost and time. Also, disposal of the kits after use by individuals was too great an issue in terms of infrastructure. We therefore decided to target it for use by local authorities so that water can be tested in different regions. | |
| - | + | ||
| - | We considered the benefits of targeting the detection kit for use by organisations instead of domestic use. One advantage is that training in the use of the kit would only need to be given to employees of the organisations, and not to whole communities, thus making the system much more viable in terms of cost and time. Also, disposal of the kits after use was too great an issue in terms of infrastructure. We therefore decided to target it for use by local authorities so that water can be tested in different regions. | + | |
Our project involves taking genes from a pathogenic bacterium, ''Streptococcus pneumoniae'', which is by no means ideal. However, an alternative could not be found in a non-pathogenic species. These genes are only involved in the competence pathway and not in any pathways that may confer pathogenicity. There are also homologous genes found in non-pathogenic bacteria. Therefore, we do not expect any adverse consequences from using these genes out of context, and we would ensure that testing is done prior to the marketing of our product so that it is certain that no pathogenicity is transferred to our bacterium. | Our project involves taking genes from a pathogenic bacterium, ''Streptococcus pneumoniae'', which is by no means ideal. However, an alternative could not be found in a non-pathogenic species. These genes are only involved in the competence pathway and not in any pathways that may confer pathogenicity. There are also homologous genes found in non-pathogenic bacteria. Therefore, we do not expect any adverse consequences from using these genes out of context, and we would ensure that testing is done prior to the marketing of our product so that it is certain that no pathogenicity is transferred to our bacterium. | ||
| - | + | We chose ''B. subtilis'' as our chassis as it is a non-pathogenic bacterium that is present in many environments, such as soil. Using ''B. subtilis'' means that the cells can be transported in spore form, allowing them to withstand large temperature extremes. This means that the whole kit will be easy to store and transport, and so will be straightforward to use in resource-poor settings. Before use, the spores would need to be germinated, but this represents no great problem. | |
Another advantage of using ''B. subtilis'' is that we can remove antibiotic resistance genes using Dif sequences, so the final synthetic organism will not be resistant to antibiotics. These antibiotic resistance genes are essential for selection during cloning. During the assembly of our constructs, we used two different antibiotics to select for cells that contained plasmids that contained either the spectinomycin or chloramphenicol resistance genes. Either side of these resistance gene casettes are Dif sites. When the constructs are integrated into the ''B. subtilis'' genome, they are relatively stable and cannot be lost, so the resistance genes are no longer needed. At this time, the Dif sites are targeted by an inherent enzyme which all ''B. subtilis'' cell possess, namely a recombinase. This recombinase removes the stretch of DNA between each Dif site, including the antibiotic resistance genes. | Another advantage of using ''B. subtilis'' is that we can remove antibiotic resistance genes using Dif sequences, so the final synthetic organism will not be resistant to antibiotics. These antibiotic resistance genes are essential for selection during cloning. During the assembly of our constructs, we used two different antibiotics to select for cells that contained plasmids that contained either the spectinomycin or chloramphenicol resistance genes. Either side of these resistance gene casettes are Dif sites. When the constructs are integrated into the ''B. subtilis'' genome, they are relatively stable and cannot be lost, so the resistance genes are no longer needed. At this time, the Dif sites are targeted by an inherent enzyme which all ''B. subtilis'' cell possess, namely a recombinase. This recombinase removes the stretch of DNA between each Dif site, including the antibiotic resistance genes. | ||
| - | We considered different types of output, such as an odour or light. However, the most reliable and the fastest way of getting a response appeared to be by using the C2,3O reaction, which has a colour output. Our only concern about this | + | We considered different types of output, such as an odour or light. However, the most reliable and the fastest way of getting a response appeared to be by using the C2,3O reaction, which has a colour output. Our only concern about this was that when testing water with a high level of sediment, it may be hard to observe the colour change, which is yellow-orange. If this were the case, an odour might be a possible output. |
The design of the final product would depend on whether it is more appropriate to test a fixed volume of water using a container, or to use a dipstick to test a variable amount of water. The latter technique would allow one to increase the sensitivity of the test by attracting cercariae using certain lipids, which could be released by the kit. This would lead to the establishment of a concentration gradient of lipids, leading to a higher number of cercaria to be detected. | The design of the final product would depend on whether it is more appropriate to test a fixed volume of water using a container, or to use a dipstick to test a variable amount of water. The latter technique would allow one to increase the sensitivity of the test by attracting cercariae using certain lipids, which could be released by the kit. This would lead to the establishment of a concentration gradient of lipids, leading to a higher number of cercaria to be detected. | ||
| Line 124: | Line 157: | ||
Due to the modular design of the detection kit, there are many other applications that would benefit from a fast response module. There are also many other parasites that could potentially be detected, especially those that use proteases to infect their host, such as hookworm, guinea worm and strongyloides. These could be other potential applications of our system. | Due to the modular design of the detection kit, there are many other applications that would benefit from a fast response module. There are also many other parasites that could potentially be detected, especially those that use proteases to infect their host, such as hookworm, guinea worm and strongyloides. These could be other potential applications of our system. | ||
| - | We decided to get some prototypes made, as this might help inform our design, thus improving the efficiency of our detection kit. Below are a few sketches | + | |} |
| - | + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | |
| - | [[Image: | + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Implementation of the detection kit: Building prototypes influenced our design |
| - | + | |- | |
| - | + | |We decided to get some prototypes made, as this might help inform our design, thus improving the efficiency of our detection kit. Below are a few sketches that Tom Jay, who studies product design, came up with. | |
| - | [[Image: | + | |} |
| - | + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:0px;" cellspacing="20" | |
| - | + | |- | |
| - | + | |[[Image:IC_Concept_1.jpg|thumb|center|290px|alt=A|This is the first design that was presented to us. ]] | |
| - | + | |[[Image:IC_prototype1.jpg|thumb|center|280px|alt=A|After receiving our feedback, the designer presented the second concept.]] | |
| - | + | |- | |
| - | + | |[[Image:IC_prototype2.jpg|thumb|center|300px|alt=A|This is our favourite design.]] | |
| - | + | |[[Image:IC_Concept2.jpg|thumb|center|300px|alt=A|Here is a prototype of the design.]] | |
| - | + | |} | |
| - | + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | |
| - | + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|References | |
| - | + | |- | |
| - | + | | | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Coon, D. R. (2005) Schistosomiasis: overview of the history, biology, clinicopathology, and laboratory diagnosis, Clinical Microbiology Newsletter 27:163–169 | Coon, D. R. (2005) Schistosomiasis: overview of the history, biology, clinicopathology, and laboratory diagnosis, Clinical Microbiology Newsletter 27:163–169 | ||
| - | |||
| - | |||
Fenwick, A. (2009) Host-parasite relations and implications for control. Advances in Parasitology. 68:247-261 | Fenwick, A. (2009) Host-parasite relations and implications for control. Advances in Parasitology. 68:247-261 | ||
| Line 201: | Line 184: | ||
Hotez, P. J., Ottesen, E., Fenwick, A., & Molyneux, D. H. (2006) The neglected tropical diseases: The ancient afflictions of stigma and poverty and the prospects for their integrated control and elimination. Hot topics in infection and immunity in children III. New York: Kluwer Academic/Plenum Publishers. | Hotez, P. J., Ottesen, E., Fenwick, A., & Molyneux, D. H. (2006) The neglected tropical diseases: The ancient afflictions of stigma and poverty and the prospects for their integrated control and elimination. Hot topics in infection and immunity in children III. New York: Kluwer Academic/Plenum Publishers. | ||
| - | |||
| - | |||
Molyneux, D. H, Hotez, P. J. & Fenwick, A. (2005) Rapid-Impact Interventions: How a Policy of Integrated Control for Africa’s Neglected Tropical Diseases Could Benefit the Poor. PLoS Medicine. 2:1064-1070 | Molyneux, D. H, Hotez, P. J. & Fenwick, A. (2005) Rapid-Impact Interventions: How a Policy of Integrated Control for Africa’s Neglected Tropical Diseases Could Benefit the Poor. PLoS Medicine. 2:1064-1070 | ||
| Line 213: | Line 194: | ||
Schmidt, M. (2008) Diffusion of synthetic biology: a challenge to biosafety Systems and Synthetic Biology | Schmidt, M. (2008) Diffusion of synthetic biology: a challenge to biosafety Systems and Synthetic Biology | ||
| - | |||
| - | Steinmann, P.,Keiser, J., Bos, R., Tanner, M. & Utzinger, J. (2005) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk, Lancet Infect. Dis. 6 pp. 411–425 | + | Steinmann, P., Keiser, J., Bos, R., Tanner, M. & Utzinger, J. (2005) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk, Lancet Infect. Dis. 6 pp. 411–425 |
| - | + | Sturrock, R. F. (1993) The control of schistosomiasis. Second report of the WHO expert committee. Geneva: World Health Organisation Vol II | |
| - | + | ||
| - | Sturrock, R. F. (1993) The control of schistosomiasis. Second report of the WHO expert committee Geneva: World Health Organisation Vol II | + | |
World Health Organisation (2003) Communicable diseases 2002: global defence against the infectious disease threat (ed. Kindhauser, M. K.). Geneva | World Health Organisation (2003) Communicable diseases 2002: global defence against the infectious disease threat (ed. Kindhauser, M. K.). Geneva | ||
| Line 226: | Line 204: | ||
WHO/UNICEF (2010) Progress on sanitation and drinking-water 2010 update | WHO/UNICEF (2010) Progress on sanitation and drinking-water 2010 update | ||
| - | |||
| - | |||
WHO (2007) Manuscript of Neglected Tropical Diseases Annual Report 2006. Geneva | WHO (2007) Manuscript of Neglected Tropical Diseases Annual Report 2006. Geneva | ||
|} | |} | ||
Latest revision as of 03:05, 28 October 2010
| Human Practices | Overview | Panel | Workshop | Meetings | Report | Documentary |
| Human practices has been a hugely important influence in the design of our final product. We contacted a number of experts very early on in the design process to allow us to truly adapt our specifications to meet the requirements of a field testing kit for parasites. This has ensured that our design is as feasible and useful as possible. | |
| Human Practices Report |
| Our project focuses on the design of a system that gives a rapid response, because we noticed that many previous biosensor projects had only achieved relatively slow responses to a stimulus. Our concept is an adaptable, modular and robust fast response system, which is fundamental for modern applications in synthetic biology.
One possible application for this fast response module is in the detection of water-borne parasites. We realised that synthetic biology is rarely used in the fight against neglected tropical diseases (NTDs), and we wanted to be pioneers in this field. Over 1 billion people - a sixth of the world’s population - suffer from one or more NTDs (WHO, 2007). Despite affecting over 90% of the world’s population in one way or another, only 10% of worldwide health research funding goes into finding preventative or curative treatment for NTDs. This statistic was published by the Global Health Forum and is now referred to as the ‘10/90 gap’ (Global Forum for Health Research, 1999). The 10/90 gap probably exists because of the fact that the majority of people affected by NTDs live in developing countries (Hotez et al, 2006), and so profit margins for NTD drugs are negligible. Therefore, Western pharmaceutical companies are unlikely to see countries in which NTDs are endemic as high priority commercial markets (Hotez et al, 2006). The World Health Organisation lists the following diseases as the main NTDs (WHO, 2007):
|
| Schistosomiasis |
| Schistosomiasis, also known as bilharzia, is an NTD that affects over 200 million people worldwide (Steinmann et al, 2005) and has drastic effects on the economic viability of the country in which it is endemic (Sturrock, R. F., 1993). The Schistosoma species are human parasites found in sub-Saharan Africa, Latin America and South-East Asia (Liu et al, 2010). The parasite has a complicated lifecycle, involving two hosts; freshwater snails and humans, and infects humans by breaking through the skin during contact with contaminated water (Gryseels et al, 2006; Coon et al, 2005).
The construction of man-made water systems has extended snail habitats by creating favourable conditions for their survival and reproduction. As people move closer to lakes, dams and other irrigations systems an optimal environment for the transmission of the parasite results, thus making schistosomiasis even more of a major global health problem (Fenwick, 2009). In recent years, the treatment of schistosomiasis, has improved markedly due to various schemes of chemotherapy, including the administration of praziquantel (Fenwick, 2009). This drug is effective as a single dose, can be administered by primary healthcare teams and directly affects the Schistosoma parasite (Coon, 2005). However, it should be noted that the primary aim of these schemes is to reduce morbidity rather than prevent transmission. At present, most of the detection of Schistosoma is only possible after it has infected the human host. There are two main types of diagnosis; antibody detection in blood serum, or egg detection in urine and stool samples (Liu et al, 2010). The latter is more commonly used but it is a lengthy and uneconomical technique. The detection of Schistosoma in the local environment is done rarely, as it is time-consuming and can require cumbersome equipment. Another drawback is the low sensitivity of the tests. The infective form of Schistosoma, called a cercaria, secretes proteases upon detection of human skin lipids (McKerrow & Salter, 2002). These proteases enable the cercariae to break through the skin barrier and infect the host. We intend to use these proteases to detect the cercariae, because they can trigger a synthetic signaling pathway in our bacterium. We have discussed a wide range of ethical, legal and social implications (ELSIs) of our project in a Human Practices Workshop, an Interdisciplinary Human Practices Panel Discussion and with [various experts in the field. This has allowed us to ensure that our design specifcaitons are influenced by Human Practices.
|
| Misuse |
| As with any scientific development, there is always the potential for misuse. We have debated who we think should have access to methods used in synthetic biology and in particular, our product. However, using synthetic biology in its current state requires huge amounts of expertise which is only possessed by research institutions and some commercial companies. Nevertheless, it has been proposed that some military organisations may possess sufficient resources to be capable of manipulating some developments for potentially detrimental ends, which might be one reason why funding for biodefence research has rocketed since 9/11 (Schmidt, 2008).
As synthetic biology develops as a field, the ease with which systems are put together and the level of automation will improve, and thus entry barriers will diminish. Technology will become more affordable, especially as there is an ‘open source’ ethos within the field of synthetic biology (this will be further discussed in ‘Legal Factors’ and 'Political Factors and Regulation', where we propose an independent, international governing body for synthetic biology). |
| Biosafety and Environmental Risks |
| Of particular concern is the uncontrolled release of Genetically Modified Organisms (GMOs), either intentional or accidental, into the environment. The prospect of contaminating groundwater is particularly alarming, especially in locations where coordination of water treatment is under-developed, as might be the case in some developing countries. We have considered how uncontrolled release would affect the local environment, and the biodiversity of its ecosystem. This led us to opt for a chassis that was already endemic in most environments. Our choice of chassis was B. subtilis, which is a bacterium that is often found in soil and is non-pathogenic.
For more information, please see our Safety Page.
To make the detection module we will integrate the required genes as two separate pieces of DNA (cassettes) directly into the B. subtilis genome. In order to limit the lifetime of the bacterium in the environment, we will integrate the cassettes at areas in the genome such that they will interfere with essential genes. One of these essential genes is needed to metabolise complex sugars (amyE) and the other is required for pyrimidine biosynthesis (pyrD), which are required for DNA synthesis. Additionally, all lab strains of B. subtilis are tryptophan knock-outs. As a result, the final version of our product would only be able to survive in special conditions where these additional nutrients are provided. Therefore, if it were to be released into the environment, it would, in theory, only survive for a limited period of time (this would be tested experimentally to quantify the exact lifetime of the bacterium in different environments and conditions). These vectors are now on the Registry and available for use. Removal of antibiotic genes using Dif excision Most techniques of introducing new genes into organisms require additional selection genes; these usually confer resistance to a particular antibiotic such as spectinomycin. Our design will also include these genes, however additional Dif sequences built into our design will ensure that these genes are quickly removed from the bacterium, using an inherent control mechanism. As a result, the bacteria used in our final product will not be antibiotic resistant. Integration directly into the genome also ensures that the detection module genes will remain in the organism long enough for the kit to be used effectively.
An additional level of confidence is that a component of the reaction mixture, called catechol, actually kills the bacteria a few hours after it is added. We have tested this and results can be found in Results. Nevertheless, we are aware that in order for this to occur, the catechol must be added appropriately, and this relies on the correct use of the detection kit. However, if the bacteria were released into the environment without being exposed to catechol, the original genome disruption would be sufficient to ensure that the bacteria would not survive.
We advocate continued, regular testing of the bacteria in order to ensure that any mutations, which would result in the deviation from its function, are noticed early and so the problem can be remediated. We would ensure that there are thorough, controlled and longitudinal studies of the effects of the bacteria on a range of environments and seasons, prior to the marketing of the product. |
| Political Factors and Regulation |
| Regulation of the use of synthetic biology is a huge concern of both synthetic biologists and the wider public. We have debated whether regulation should be external or internal, and government-led or –funded. Self-regulation could be an effective way of ensuring that rules are adhered to, but with a lack of external scrutiny, it is unlikely to be sufficient for the public to feel entirely confident about the issues raised. This is due to the difficulty in keeping track of such a diffuse network of researchers (Schmidt, 2008) and the fact that there is always the potential for people to ignore the rules. We therefore believe that an international, independent body should monitor organisations working in the field of synthetic biology, and that there should be transparency in all their dealings.
We wanted to know what control measures were in place at our university and whether or not the government plays a role in this. At Imperial College London, the GM safety committee is responsible for ensuring that all research adheres to the regulations set down by the Health and Safety Executive (HSE). The first stage in the commencement of a project is the completion of a comprehensive risk assessment, which is submitted to the GM safety committee, which will then (i) approve the risk assessment and (ii) identify control measures. These control measures then need to be put in place. If the GM classification is Class 2 or higher, the project must be discussed with the Director of Occupational Health to determine whether health surveillance is necessary. |
| Biosafety and Environmental Risks |
| A legal framework to control the use and ownership of the developments of synthetic biology is essential in order to establish the open source philosophy. We are aware that copyrights and patents can limit the scope of further research, because information is not accessible or research centres must pay heavily to use such innovations. There are many arguments for the implementation of open source synthetic biology, as opposed to using patenting laws. It has been suggested that open source projects drive innovation within the field, but they can also present huge logistical problems in terms of regulation upon diffusion of the technology (Schmidt, 2008).
There has been extensive media coverage concerning access to medicines and technology in the fight against NTDs. In the majority of cases, strong intellectual property rights (IPR) regulations rule out the generic production of drugs so that no alternatives to costly medicines are available. The majority of NTDs occur in developing countries, where healthcare provision is often poor and there are few resources available in terms of NTD medication. Strong IPR regulations for NTD drugs are often seen as unnecessary because pharmaceutical companies will not generate profits because people who can afford the drugs do not need them, and vice versa. Wider access to NTD drugs could be achieved by ensuring that research is available through open source.
|
| Economic factors |
| We have ensured that the cost of production of the detection kit is low enough to make it affordable to the people who need it. This was achieved by designing a product that is economical to manufacture and uses inexpensive chemicals in the process.
The role of industry in synthetic biology is a controversial one. Industry is driven by profit, and we would like to ensure that no company has a monopoly in any particular area through the ownership of one or more of our modules. Ideally, we would like the technology to be available for the manufacturing of generic products. In particular, universities are centres of learning, and are not profit-generating businesses. We therefore believe that it is essential that research done at universities is accessible and can be used to benefit others. This could be achieved by making the modules available through open source, or by licensing the modules to different companies so that no one company has a monopoly. |
| Social factors |
It is very important to us that the communities who would benefit from the detection kits have access to them and so they must be affordable and available in appropriate locations. Accessibility to the detection kits would depend on the following factors:
We do not believe that our detection kit on its own can solve the problem of Schistosoma. Improved sanitation and education is needed in combination with the environmental control of parasites. It would be necessary to carry out an assessment of the level of education regarding parasitic infections, in particular schistosomiasis, in order to determine where further education was needed. This education programme could be given alongside the training in the use of the detection kit to ensure the long-term feasibility of our project, and also to overcome any cultural boundaries there may be. It is also essential that we determine the social acceptability of the kits before we finalise the design. During this process, it is possible that we would find that different designs are more appropriate in different settings, and the overall design may be needed to be tailored to a specific community, depending on the setting in which it is implemented. The benefits to local communities are unlikely to be seen straight away. This is due to the nature of NTDs and their prolonged effects on individuals, and the fact that much of the problem with them is due to socio-economic factors (Hotez et al, 2006; Molyneux et al, 2005). However, as the incidence of, for example, schistosomiasis decreases, the economic productivity of a particular population will hopefully improve, and this should result in a positive feedback loop where the NTD incidence decreases even further due to the improved economy of the given community.
|
| Design Specifications |
| Due to the constraints imposed by the environment in which most NTDs occur, our detection kit needs to be portable to resource-poor settings, affordable, easy to use and store, and not dangerous if released into the environment.
We considered the benefits of targeting the detection kit for use by organisations instead of domestic use. One advantage is that training in the use of the kit would only need to be given to employees of the organisations, and not to whole communities, thus making the system much more viable in terms of cost and time. Also, disposal of the kits after use by individuals was too great an issue in terms of infrastructure. We therefore decided to target it for use by local authorities so that water can be tested in different regions. Our project involves taking genes from a pathogenic bacterium, Streptococcus pneumoniae, which is by no means ideal. However, an alternative could not be found in a non-pathogenic species. These genes are only involved in the competence pathway and not in any pathways that may confer pathogenicity. There are also homologous genes found in non-pathogenic bacteria. Therefore, we do not expect any adverse consequences from using these genes out of context, and we would ensure that testing is done prior to the marketing of our product so that it is certain that no pathogenicity is transferred to our bacterium. We chose B. subtilis as our chassis as it is a non-pathogenic bacterium that is present in many environments, such as soil. Using B. subtilis means that the cells can be transported in spore form, allowing them to withstand large temperature extremes. This means that the whole kit will be easy to store and transport, and so will be straightforward to use in resource-poor settings. Before use, the spores would need to be germinated, but this represents no great problem. Another advantage of using B. subtilis is that we can remove antibiotic resistance genes using Dif sequences, so the final synthetic organism will not be resistant to antibiotics. These antibiotic resistance genes are essential for selection during cloning. During the assembly of our constructs, we used two different antibiotics to select for cells that contained plasmids that contained either the spectinomycin or chloramphenicol resistance genes. Either side of these resistance gene casettes are Dif sites. When the constructs are integrated into the B. subtilis genome, they are relatively stable and cannot be lost, so the resistance genes are no longer needed. At this time, the Dif sites are targeted by an inherent enzyme which all B. subtilis cell possess, namely a recombinase. This recombinase removes the stretch of DNA between each Dif site, including the antibiotic resistance genes. We considered different types of output, such as an odour or light. However, the most reliable and the fastest way of getting a response appeared to be by using the C2,3O reaction, which has a colour output. Our only concern about this was that when testing water with a high level of sediment, it may be hard to observe the colour change, which is yellow-orange. If this were the case, an odour might be a possible output. The design of the final product would depend on whether it is more appropriate to test a fixed volume of water using a container, or to use a dipstick to test a variable amount of water. The latter technique would allow one to increase the sensitivity of the test by attracting cercariae using certain lipids, which could be released by the kit. This would lead to the establishment of a concentration gradient of lipids, leading to a higher number of cercaria to be detected. We intend the test to be quantitative, but this would require a spectrophotometer. However, we have looked at ways to implement quantification by eye, much like a pH indicator test. Due to the modular design of the detection kit, there are many other applications that would benefit from a fast response module. There are also many other parasites that could potentially be detected, especially those that use proteases to infect their host, such as hookworm, guinea worm and strongyloides. These could be other potential applications of our system. |
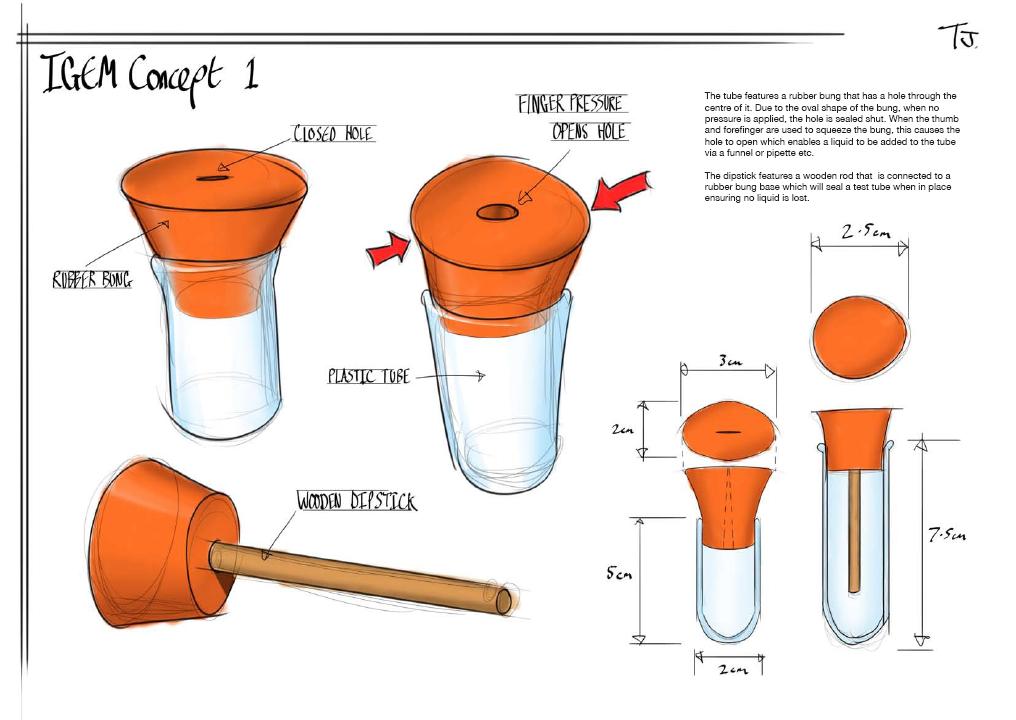
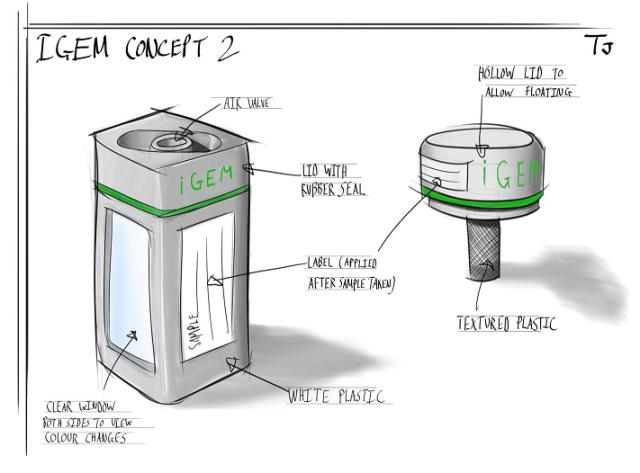
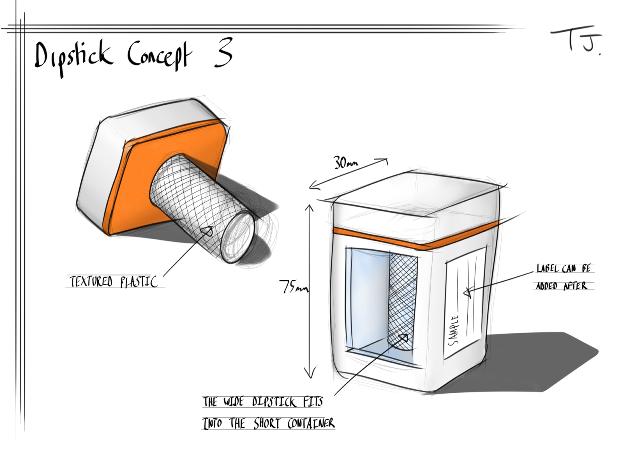
| Implementation of the detection kit: Building prototypes influenced our design |
| We decided to get some prototypes made, as this might help inform our design, thus improving the efficiency of our detection kit. Below are a few sketches that Tom Jay, who studies product design, came up with. |
| References |
|
Coon, D. R. (2005) Schistosomiasis: overview of the history, biology, clinicopathology, and laboratory diagnosis, Clinical Microbiology Newsletter 27:163–169 Fenwick, A. (2009) Host-parasite relations and implications for control. Advances in Parasitology. 68:247-261 Global Forum for Health Research (1999) 10/90 Report on Health Research 1999 Gryseels, B., Polman, K., Clerinx, J. & Kestens, L. (2006) Human schistosomiasis, Lancet 36, pp. 1106–1118 Hotez, P. J., Ottesen, E., Fenwick, A., & Molyneux, D. H. (2006) The neglected tropical diseases: The ancient afflictions of stigma and poverty and the prospects for their integrated control and elimination. Hot topics in infection and immunity in children III. New York: Kluwer Academic/Plenum Publishers. Molyneux, D. H, Hotez, P. J. & Fenwick, A. (2005) Rapid-Impact Interventions: How a Policy of Integrated Control for Africa’s Neglected Tropical Diseases Could Benefit the Poor. PLoS Medicine. 2:1064-1070 Liu, Z. S., Zhang, L., Yang, H., Zhu, Y. H., Jin, W., Song, Q. A. & Yang, X. L. (2010) Magnetic microbead-based enzyme-linked immunoassay for detection of Schistosoma japonicum antibody in human serum. Analytical Biochemistry 404:127-134 McKerrow, J. H. & Salter, J. (2002) Invasion of skin by Schistosoma cercariae. Trends in Parasitology 18:193-195 Patz, J. A., Epstein, P. R., Burke, T. A. & Balbus, J. M. (1996) Global climate change and emerging infectious diseases. Journal of the American Medical Association. 275(3):217-223 Schmidt, M. (2008) Diffusion of synthetic biology: a challenge to biosafety Systems and Synthetic Biology Steinmann, P., Keiser, J., Bos, R., Tanner, M. & Utzinger, J. (2005) Schistosomiasis and water resources development: systematic review, meta-analysis, and estimates of people at risk, Lancet Infect. Dis. 6 pp. 411–425 Sturrock, R. F. (1993) The control of schistosomiasis. Second report of the WHO expert committee. Geneva: World Health Organisation Vol II World Health Organisation (2003) Communicable diseases 2002: global defence against the infectious disease threat (ed. Kindhauser, M. K.). Geneva World Health Organisation (2007) Report of the global partners' meeting on neglected tropical dieseases. Geneva WHO/UNICEF (2010) Progress on sanitation and drinking-water 2010 update WHO (2007) Manuscript of Neglected Tropical Diseases Annual Report 2006. Geneva |
 "
"