Team:Groningen/6 September 2010
From 2010.igem.org
| (5 intermediate revisions not shown) | |||
| Line 45: | Line 45: | ||
Two expression experiments were done this week. | Two expression experiments were done this week. | ||
| + | |||
| + | '''Exression experiment''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | '''Peter & David''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | For this experiment, the following B. subtilis 168 strains were used: | ||
| + | |||
| + | <pre> | ||
| + | |||
| + | |||
| + | |||
| + | </pre> | ||
| + | |||
| + | <br> | ||
| + | |||
| + | All cultures were grown overnight at 37 degrees Celsius in a shaker room, the appropriate antibiotics were used at all points in time during this experiment. | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Overnight cultures were used to dilute to a B. subtilis culture of 0,1 OD, these strains were divided into ‘’induced’’ and ‘’non-induced’’. Induction with 0,5% subtilin was done at a OD of 0,5 (approximately 2,5 hours after growth of the 0,1 culture started). | ||
| + | |||
| + | <br> | ||
| + | |||
| + | After that the OD of the cultures was measured every .. hours. | ||
| + | |||
| + | <br> | ||
| + | |||
| + | '''Sample preperation''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | After .. hours, .. after induction, the samples were collected and processed. The following procedures were used: | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Pellet preperation ([[PelletPrepGR]]) | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Supernatant processing ([[SupernatantPrepGR]]) | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Cell disruption ([[ExtractionCellWallsGR]]) | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Lysozyme preperation ([[LysozymePrepGR]]) | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Analysis was done using SDS-PAGE ([[SDS-PAGEGR]]) and THT staining ([[THTstainingGR]]). | ||
| + | |||
| + | <br> | ||
| + | |||
| + | '''Results''': | ||
| + | |||
| + | <br> | ||
| + | |||
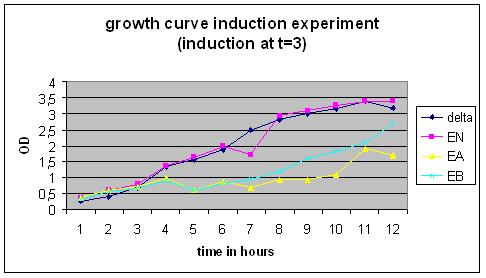
| + | '''Growth Curve''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | [[Image:GrowthCurveGR3.jpg|400px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
| + | <br> | ||
| + | |||
| + | '''THT Staining''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | [[Image:THTGR3.jpg|400px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| + | <br> | ||
| + | |||
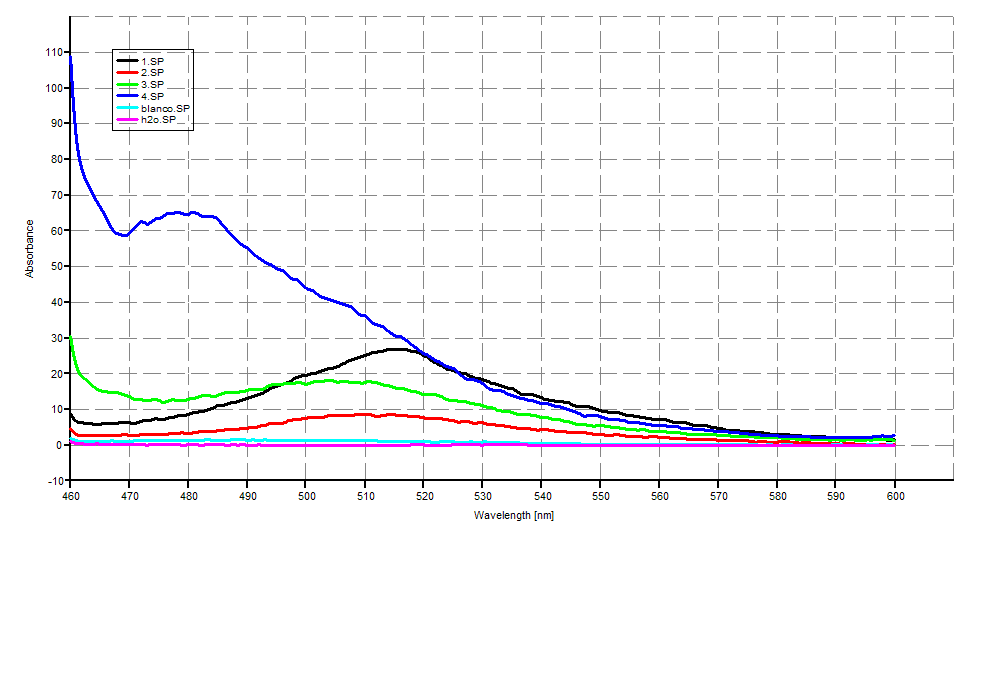
| + | '''SDS-PAGE''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | [[Image:SDS-PAGEGR3.jpg|400px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | <br> | ||
| + | |||
| + | '''Exression experiment''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | '''Peter & David''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | For this experiment, the following B. subtilis 168 strains were used: | ||
| + | |||
| + | <pre> | ||
| + | |||
| + | |||
| + | |||
| + | </pre> | ||
| + | |||
| + | <br> | ||
| + | |||
| + | All cultures were grown overnight at 37 degrees Celsius in a shaker room, the appropriate antibiotics were used at all points in time during this experiment. | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Overnight cultures were used to dilute to a B. subtilis culture of 0,1 OD, these strains were divided into ‘’induced’’ and ‘’non-induced’’. Induction with 0,5% subtilin was done at a OD of 0,5 (approximately 2,5 hours after growth of the 0,1 culture started). | ||
| + | |||
| + | <br> | ||
| + | |||
| + | After that the OD of the cultures was measured every .. hours. | ||
| + | |||
| + | <br> | ||
| + | |||
| + | '''Sample preperation''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | After .. hours, .. after induction, the samples were collected and processed. The following procedures were used: | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Pellet preperation ([[PelletPrepGR]]) | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Supernatant processing ([[SupernatantPrepGR]]) | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Cell disruption ([[ExtractionCellWallsGR]]) | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Lysozyme preperation ([[LysozymePrepGR]]) | ||
| + | |||
| + | <br> | ||
| + | |||
| + | Analysis was done using SDS-PAGE ([[SDS-PAGEGR]]) and THT staining ([[THTstainingGR]]). | ||
| + | |||
| + | <br> | ||
| + | |||
| + | '''Results''': | ||
| + | |||
| + | <br> | ||
| + | |||
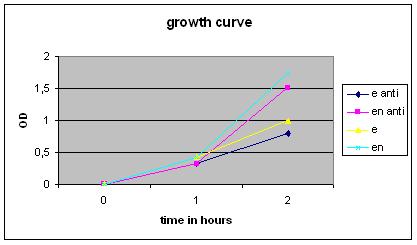
| + | '''Growth Curve''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | [[Image:GrowthCurveGR4.jpg|400px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| + | |||
| + | <br> | ||
| + | |||
| + | '''THT Staining''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | [[Image:THTGR4.jpg|400px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| + | <br> | ||
| + | |||
| + | '''SDS-PAGE''' | ||
| + | |||
| + | <br> | ||
| + | |||
| + | [[Image:SDS-PAGEGR4.jpg|400px]] | ||
| + | |||
| + | <br> | ||
| + | |||
| + | <br> | ||
| + | |||
| + | |||
| Line 50: | Line 242: | ||
As a follow up experiment for the last [https://2010.igem.org/Team:Groningen/23_August_2010 biofilm induction ] ''Bacillus subtilis'' wit Chaplin E and chaplin H were grown while continuously adding subtilin to keep expression up. After 3hrs of growth they where induced with 1%subtilin. Every hour an additional 0.5%subtilin was added. As a negative control uninduced strains where used. Furthermore shaking cultures (induced and uninduced) where grown simultaneously to check OD levels. Only the chaplin E strain showed reduced growth, but no differences other than retarded growth were visible. | As a follow up experiment for the last [https://2010.igem.org/Team:Groningen/23_August_2010 biofilm induction ] ''Bacillus subtilis'' wit Chaplin E and chaplin H were grown while continuously adding subtilin to keep expression up. After 3hrs of growth they where induced with 1%subtilin. Every hour an additional 0.5%subtilin was added. As a negative control uninduced strains where used. Furthermore shaking cultures (induced and uninduced) where grown simultaneously to check OD levels. Only the chaplin E strain showed reduced growth, but no differences other than retarded growth were visible. | ||
| + | |||
| + | '''Modellers:''' Work on gene expression model ''Joël, Djoke, Laura'' Work on information standard ''Arend'' | ||
Latest revision as of 20:12, 25 October 2010
Assembly of biobricks in pSB1C3-backbone for submission to parts registry by Maarten
Control restrictions with EcoRI and PstI on constructs:
| 2 µL construct |
| 2 µL buffer O |
| 0,5 µL EcoRI |
| 0,5 µL PstI |
| 15 µL MQ |
| Construct | Expected band sizes (bp) |
|---|---|
| pSB1C3-C | 2031, 848 |
| pSB1C3-DC | 2031, 872 |
| pSB1C3-E | 2031, 317 |
| pSB1C3-H | 2031, 302 |
| pSB1C3-Sortase | 2031, 689 |
| pSB1C3-RFP | 2031, 1069 |
The gel that was run did not show any DNA present on the gel. Experiment was continued on 8 September.
Expression experiments -David & Peter
Two expression experiments were done this week.
Exression experiment
Peter & David
For this experiment, the following B. subtilis 168 strains were used:
All cultures were grown overnight at 37 degrees Celsius in a shaker room, the appropriate antibiotics were used at all points in time during this experiment.
Overnight cultures were used to dilute to a B. subtilis culture of 0,1 OD, these strains were divided into ‘’induced’’ and ‘’non-induced’’. Induction with 0,5% subtilin was done at a OD of 0,5 (approximately 2,5 hours after growth of the 0,1 culture started).
After that the OD of the cultures was measured every .. hours.
Sample preperation
After .. hours, .. after induction, the samples were collected and processed. The following procedures were used:
Pellet preperation (PelletPrepGR)
Supernatant processing (SupernatantPrepGR)
Cell disruption (ExtractionCellWallsGR)
Lysozyme preperation (LysozymePrepGR)
Analysis was done using SDS-PAGE (SDS-PAGEGR) and THT staining (THTstainingGR).
Results:
Growth Curve
THT Staining
SDS-PAGE
Exression experiment
Peter & David
For this experiment, the following B. subtilis 168 strains were used:
All cultures were grown overnight at 37 degrees Celsius in a shaker room, the appropriate antibiotics were used at all points in time during this experiment.
Overnight cultures were used to dilute to a B. subtilis culture of 0,1 OD, these strains were divided into ‘’induced’’ and ‘’non-induced’’. Induction with 0,5% subtilin was done at a OD of 0,5 (approximately 2,5 hours after growth of the 0,1 culture started).
After that the OD of the cultures was measured every .. hours.
Sample preperation
After .. hours, .. after induction, the samples were collected and processed. The following procedures were used:
Pellet preperation (PelletPrepGR)
Supernatant processing (SupernatantPrepGR)
Cell disruption (ExtractionCellWallsGR)
Lysozyme preperation (LysozymePrepGR)
Analysis was done using SDS-PAGE (SDS-PAGEGR) and THT staining (THTstainingGR).
Results:
Growth Curve
THT Staining
SDS-PAGE
Continous induction of chaplins
As a follow up experiment for the last biofilm induction Bacillus subtilis wit Chaplin E and chaplin H were grown while continuously adding subtilin to keep expression up. After 3hrs of growth they where induced with 1%subtilin. Every hour an additional 0.5%subtilin was added. As a negative control uninduced strains where used. Furthermore shaking cultures (induced and uninduced) where grown simultaneously to check OD levels. Only the chaplin E strain showed reduced growth, but no differences other than retarded growth were visible.
Modellers: Work on gene expression model Joël, Djoke, Laura Work on information standard Arend
 "
"