Team:WITS-South Africa/The Machine
From 2010.igem.org
LauraMillroy (Talk | contribs) |
|||
| Line 10: | Line 10: | ||
<div class="heading"> | <div class="heading"> | ||
| - | '''Machine Schematic''' | + | |
| + | == '''Machine Schematic''' == | ||
| + | |||
</div> | </div> | ||
| Line 16: | Line 18: | ||
<div class="heading"> | <div class="heading"> | ||
| - | '''Cassette 1 in population 1''' | + | |
| + | == '''Cassette 1 in population 1''' == | ||
| + | |||
</div> | </div> | ||
| Line 22: | Line 26: | ||
<div class ="heading"> | <div class ="heading"> | ||
| - | Lac/AraC Promoter | + | |
| + | === Lac/AraC Promoter === | ||
| + | |||
</div> | </div> | ||
The Lac/AraC promoter will be a synthetic fusion promoter comprised of the operator from the arabinose operon and the Lac promoter from the lac operon. The AraC protein is constitutively expressed and binds to the arabinose operon in the absence of the arabinose sugar. When arabinose is present, or an isomer thereof, it induces a conformational change in AraC thus preventing it from binding the operon and thus allowing transcription. Since IPTG is an isomer of β-galactosidase, it will induce the same conformational effect on AraC thus inducing transcription. In this way, the exogenous addition of IPTG will serve as a proxy for the HPV virus and induce the transcription cassette 1. Furthermore, as illustrated by Lutz and Bujard, the degree of induction is influenced by both IPTG and arabinose. Hence, promoter activity can be regulated and finely tuned by the addition of varying concentrations of IPTG and arabinose. The Lac/AraC promoter will be synthesized by primer-primer annealing and subsequent PCR elongation. | The Lac/AraC promoter will be a synthetic fusion promoter comprised of the operator from the arabinose operon and the Lac promoter from the lac operon. The AraC protein is constitutively expressed and binds to the arabinose operon in the absence of the arabinose sugar. When arabinose is present, or an isomer thereof, it induces a conformational change in AraC thus preventing it from binding the operon and thus allowing transcription. Since IPTG is an isomer of β-galactosidase, it will induce the same conformational effect on AraC thus inducing transcription. In this way, the exogenous addition of IPTG will serve as a proxy for the HPV virus and induce the transcription cassette 1. Furthermore, as illustrated by Lutz and Bujard, the degree of induction is influenced by both IPTG and arabinose. Hence, promoter activity can be regulated and finely tuned by the addition of varying concentrations of IPTG and arabinose. The Lac/AraC promoter will be synthesized by primer-primer annealing and subsequent PCR elongation. | ||
| Line 31: | Line 37: | ||
<div class="heading"> | <div class="heading"> | ||
| - | PlcR-PapR Quorum Molecule | + | |
| + | === PlcR-PapR Quorum Molecule === | ||
| + | |||
</div> | </div> | ||
The PlcR regulon in Bacillus thuringiensis and Bacillus cereus houses approximately 15 genes required for the production of many virulence factors. The activation of this regulon is under the control of the PlcR peptide which binds a region within the promoter known as the PlcR box. The papR gene - which is found within the PlcR regulon - encodes a 48 amino acid peptide that is crucial for the binding to and subsequent activation of the PlcR box. The PapR pro-peptide is secreted from the cell (via the SecA pathway) following translation, cleaved extra-cellularly and the resulting pentapeptide is re-imported into the cell via an oligopeptide permease (a protease ubiquitous to the extra-cellular matrix of Gram-positive bacteria). Once inside the cell, PapR allows PlcR to bind to the PlcR box thus activating the regulon. Due to the presence of the plcR gene within the regulon, PlcR positively regulates its own transcription. The PlcR regulon within Bacillus anthracis houses a gene that codes for a PlcR-PapR fusion protein which was found to strongly induce transcription of the native plcR gene. It has been shown, by Pomerantsev and colleagues, that binding to the PlcR box and activation of the PlcR regulon is achieved by the expression of a hetrologous PlcR-PapR fusion protein. So as to ectopically express both PlcR and PapR in Lactobacillus gasseri, a sequence coding for the PlcR-PapR fusion protein will be derived from bioinformatic analysis of the native sequence in Bacillus anthracis. This will be implemented due to the inherent compactness of the fusion protein as well as ease of transportability when inserting into a foreign bacteria. The plcR-papR sequence will be synthesized by Gene Art and furthermore, the sequence will be optimized by an online tool. | The PlcR regulon in Bacillus thuringiensis and Bacillus cereus houses approximately 15 genes required for the production of many virulence factors. The activation of this regulon is under the control of the PlcR peptide which binds a region within the promoter known as the PlcR box. The papR gene - which is found within the PlcR regulon - encodes a 48 amino acid peptide that is crucial for the binding to and subsequent activation of the PlcR box. The PapR pro-peptide is secreted from the cell (via the SecA pathway) following translation, cleaved extra-cellularly and the resulting pentapeptide is re-imported into the cell via an oligopeptide permease (a protease ubiquitous to the extra-cellular matrix of Gram-positive bacteria). Once inside the cell, PapR allows PlcR to bind to the PlcR box thus activating the regulon. Due to the presence of the plcR gene within the regulon, PlcR positively regulates its own transcription. The PlcR regulon within Bacillus anthracis houses a gene that codes for a PlcR-PapR fusion protein which was found to strongly induce transcription of the native plcR gene. It has been shown, by Pomerantsev and colleagues, that binding to the PlcR box and activation of the PlcR regulon is achieved by the expression of a hetrologous PlcR-PapR fusion protein. So as to ectopically express both PlcR and PapR in Lactobacillus gasseri, a sequence coding for the PlcR-PapR fusion protein will be derived from bioinformatic analysis of the native sequence in Bacillus anthracis. This will be implemented due to the inherent compactness of the fusion protein as well as ease of transportability when inserting into a foreign bacteria. The plcR-papR sequence will be synthesized by Gene Art and furthermore, the sequence will be optimized by an online tool. | ||
| Line 40: | Line 48: | ||
<div class="heading"> | <div class="heading"> | ||
| - | Venus | + | |
| + | === Venus === | ||
| + | |||
</div> | </div> | ||
So as to assess the degree of activation of cassette 1, a variant of the Yellow Fluorescent Protein, Venus, has been included in the construct. So as to obtain quantitative data as to the efficacy of IPTG in inducing the activation of cassette 1, a host of fluorometric analysis will be performed on a cassette-containing colony. Venus, which possesses a known excitation:emission characteristic, will be used as a reporter protein to quantify the degree of transcription (by proxy of fluorescence) with use of a fluorometer. Although venus is a ubiquitous fluorescent protein found in the laboratory, specialised sequences will be ordered from Gene Art. Furthermore, the sequences will be codon optimized so as to produce the greatest protein yield and fluorometric activity in Lactobacillus gasseri. | So as to assess the degree of activation of cassette 1, a variant of the Yellow Fluorescent Protein, Venus, has been included in the construct. So as to obtain quantitative data as to the efficacy of IPTG in inducing the activation of cassette 1, a host of fluorometric analysis will be performed on a cassette-containing colony. Venus, which possesses a known excitation:emission characteristic, will be used as a reporter protein to quantify the degree of transcription (by proxy of fluorescence) with use of a fluorometer. Although venus is a ubiquitous fluorescent protein found in the laboratory, specialised sequences will be ordered from Gene Art. Furthermore, the sequences will be codon optimized so as to produce the greatest protein yield and fluorometric activity in Lactobacillus gasseri. | ||
| Line 49: | Line 59: | ||
<div class ="heading"> | <div class ="heading"> | ||
| - | Terminator | + | |
| + | === Terminator === | ||
| + | |||
</div> | </div> | ||
So as to compartmentalize the cassette and explicitly terminate transcription, the inclusion of a double terminator from the Registry of Standard Parts is included in the design. The terminator used to ‘cap’ cassette 1 is a rho-independent terminator and will inhibit polymerase functioning by the formation of a stem-loop structure. The terminator DNA will be sourced from the Standard Registry of Parts. | So as to compartmentalize the cassette and explicitly terminate transcription, the inclusion of a double terminator from the Registry of Standard Parts is included in the design. The terminator used to ‘cap’ cassette 1 is a rho-independent terminator and will inhibit polymerase functioning by the formation of a stem-loop structure. The terminator DNA will be sourced from the Standard Registry of Parts. | ||
| Line 55: | Line 67: | ||
<div class="heading"> | <div class="heading"> | ||
| - | '''Cassette 2 in population 2''' | + | |
| + | == '''Cassette 2 in population 2''' == | ||
| + | |||
</div> | </div> | ||
| Line 61: | Line 75: | ||
<div class="heading"> | <div class="heading"> | ||
| - | PlcR Promoter | + | |
| + | === PlcR Promoter === | ||
| + | |||
</div> | </div> | ||
Through the identification of the PlcR box within the promoter of the PlcR regulon, a promoter that contains the PlcR box as well the requisite transcription-initiating landmarks within the promoter will be synthesized. Hence, any construct that is placed downstream of the promoter will be activated by the addition of exogenous PlcR and the facilitating peptide PapR. The PlcR-PapR promoter will be synthesized through primer-primer annealing and subsequent PCR elongation. | Through the identification of the PlcR box within the promoter of the PlcR regulon, a promoter that contains the PlcR box as well the requisite transcription-initiating landmarks within the promoter will be synthesized. Hence, any construct that is placed downstream of the promoter will be activated by the addition of exogenous PlcR and the facilitating peptide PapR. The PlcR-PapR promoter will be synthesized through primer-primer annealing and subsequent PCR elongation. | ||
| Line 70: | Line 86: | ||
<div class="heading"> | <div class="heading"> | ||
| - | PlcR-PapR Fusion Gene | + | ===PlcR-PapR Fusion Gene=== |
</div> | </div> | ||
The PlcR-PapR fusion gene that codes for the activating peptides PlcR and PapR has been included in construct so as to amplify the initial signal presented by population 1. Since the fusion protein of PlcR and PapR is translated into its inactive form, there will be no direct self stimulatory effects within the same bacterium. Once secreted and processed, the peptides will serve to activate neighboring bacteria thus ensuring the propagation of the signal throughout the entire population. | The PlcR-PapR fusion gene that codes for the activating peptides PlcR and PapR has been included in construct so as to amplify the initial signal presented by population 1. Since the fusion protein of PlcR and PapR is translated into its inactive form, there will be no direct self stimulatory effects within the same bacterium. Once secreted and processed, the peptides will serve to activate neighboring bacteria thus ensuring the propagation of the signal throughout the entire population. | ||
| Line 79: | Line 95: | ||
<div class="heading"> | <div class="heading"> | ||
| - | Phage Activator | + | ===Phage Activator=== |
</div> | </div> | ||
Developed by the winners of 2009 iGEM competition, Team Cambridge developed an array of activator and promoter combinations (derived from bacteriophages) that have varying degrees of binding and activating strengths. The intention was to create a library of activators and promoters than can be combined in such a way so as to vary the degree of negative feedback SpooA exerts on the PlcR-PapR promoter. As such, several alternative endings of cassette 2 will be created, and based on experimental procedure a combination will be chosen so as to introduce an effective delay in repression. This delay is crucial so as to allow sufficient transcription of both the PlcR-PapR fusion peptide as well as the E. chromi pigment as this ensures adequate propagation of the signal throughout the colony as well as sufficient E. chromi being produced to ensure a distinctly visible report. The phage activator will be sourced from the Registry of Standard Parts. | Developed by the winners of 2009 iGEM competition, Team Cambridge developed an array of activator and promoter combinations (derived from bacteriophages) that have varying degrees of binding and activating strengths. The intention was to create a library of activators and promoters than can be combined in such a way so as to vary the degree of negative feedback SpooA exerts on the PlcR-PapR promoter. As such, several alternative endings of cassette 2 will be created, and based on experimental procedure a combination will be chosen so as to introduce an effective delay in repression. This delay is crucial so as to allow sufficient transcription of both the PlcR-PapR fusion peptide as well as the E. chromi pigment as this ensures adequate propagation of the signal throughout the colony as well as sufficient E. chromi being produced to ensure a distinctly visible report. The phage activator will be sourced from the Registry of Standard Parts. | ||
| Line 88: | Line 104: | ||
<div class="heading"> | <div class="heading"> | ||
| - | mCherry | + | ===mCherry=== |
</div> | </div> | ||
Akin to the function of Venus in cassette 1, mCherry - a monomeric fluorescent protein - will be used in assaying the degree of transcription induced in colony 2 by incident PlcR-PapR quorum molecules. In addition to the visual report that will be produced as the response signal that propagates throughout the colony, mCherry will aid in the quantification of the mean fluorescent response of the colony. mCherry will be sourced from the Registry of Standard Parts. | Akin to the function of Venus in cassette 1, mCherry - a monomeric fluorescent protein - will be used in assaying the degree of transcription induced in colony 2 by incident PlcR-PapR quorum molecules. In addition to the visual report that will be produced as the response signal that propagates throughout the colony, mCherry will aid in the quantification of the mean fluorescent response of the colony. mCherry will be sourced from the Registry of Standard Parts. | ||
| Line 97: | Line 113: | ||
<div class="heading"> | <div class="heading"> | ||
| - | Terminator | + | ===Terminator=== |
</div> | </div> | ||
As described above, the terminator ends transcription. By placing a terminator in the middle of cassette 2, it introduces a time delay between the activation of the initial region of cassette 2 and the feedback component, SpooA. The terminator DNA will be sourced from the Registry of Standard Parts. | As described above, the terminator ends transcription. By placing a terminator in the middle of cassette 2, it introduces a time delay between the activation of the initial region of cassette 2 and the feedback component, SpooA. The terminator DNA will be sourced from the Registry of Standard Parts. | ||
| Line 105: | Line 121: | ||
<div class="heading"> | <div class="heading"> | ||
| - | PO Phage Promoter | + | ===PO Phage Promoter=== |
</div> | </div> | ||
Forming the receptor of the cognate activator-promoter pair of phage sensitivity tuners, the PO promoter will initiate transcription when the phage activator binds the promoter. Since the genes downstream of the promoter will only be transcribed after the phage activator is transcribed, translated and folded, a time delay is inserted due to this unavoidable metabolic process. The PO promoter will be sourced from the Registry of Standard Parts. | Forming the receptor of the cognate activator-promoter pair of phage sensitivity tuners, the PO promoter will initiate transcription when the phage activator binds the promoter. Since the genes downstream of the promoter will only be transcribed after the phage activator is transcribed, translated and folded, a time delay is inserted due to this unavoidable metabolic process. The PO promoter will be sourced from the Registry of Standard Parts. | ||
| Line 113: | Line 129: | ||
<div class="heading"> | <div class="heading"> | ||
| - | E. chromi | + | ===E. chromi=== |
</div> | </div> | ||
So as to produce a chromogenic indication of the presence of HPV (or a proxy thereof), the E. chromi pigment will be used. The E. chromi biobrick is a composite biobrick comprised of sequential enzymes that process a substrate until a visible dye is produced. The combination of four sequential enzymes converts the substrate farnesyl pyrophosphate (which is colourless) to the pigment beta-carotine via several intermediates. The E. chromi biobrick will be sourced from the Registry of Standard Parts. | So as to produce a chromogenic indication of the presence of HPV (or a proxy thereof), the E. chromi pigment will be used. The E. chromi biobrick is a composite biobrick comprised of sequential enzymes that process a substrate until a visible dye is produced. The combination of four sequential enzymes converts the substrate farnesyl pyrophosphate (which is colourless) to the pigment beta-carotine via several intermediates. The E. chromi biobrick will be sourced from the Registry of Standard Parts. | ||
| Line 122: | Line 138: | ||
<div class="heading"> | <div class="heading"> | ||
| - | SpooA | + | ===SpooA=== |
</div> | </div> | ||
So as to provide a suitable negative feedback loop to the ‘machine’, SpooA is a protein that binds to the regions adjacent to the PlcR box in the PlcR-PapR promoter. Once produced, the repression of SpooA will negate the production of both mCherry as well as E. chromi. Furthermore, SpooA will inhbit it’s own production this derepressing cassette 2 in such a way that the system, if left to its own devices in the presence of a suitable concentration of PlcR-PapR, will exhibit oscillatory behavior. SpooA will be synthesized by Gene Art and will be codon optimized for Lactobacillus gasseri so as to ensure optimal production and expression. | So as to provide a suitable negative feedback loop to the ‘machine’, SpooA is a protein that binds to the regions adjacent to the PlcR box in the PlcR-PapR promoter. Once produced, the repression of SpooA will negate the production of both mCherry as well as E. chromi. Furthermore, SpooA will inhbit it’s own production this derepressing cassette 2 in such a way that the system, if left to its own devices in the presence of a suitable concentration of PlcR-PapR, will exhibit oscillatory behavior. SpooA will be synthesized by Gene Art and will be codon optimized for Lactobacillus gasseri so as to ensure optimal production and expression. | ||
| Line 130: | Line 146: | ||
<div class="heading"> | <div class="heading"> | ||
| - | Terminator | + | ===Terminator=== |
</div> | </div> | ||
So as to provide a final cap to cassette 2 and the machine in whole, a terminator will be placed to end transcription. The terminator will be sourced from the Registry of Standard Parts. | So as to provide a final cap to cassette 2 and the machine in whole, a terminator will be placed to end transcription. The terminator will be sourced from the Registry of Standard Parts. | ||
Revision as of 14:07, 13 October 2010

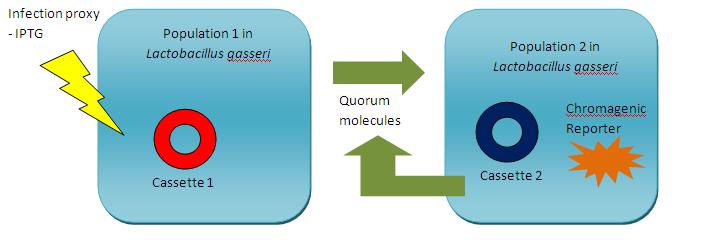
Model Outline
Contents |
Machine Schematic
Cassette 1 in population 1
Lac/AraC Promoter
The Lac/AraC promoter will be a synthetic fusion promoter comprised of the operator from the arabinose operon and the Lac promoter from the lac operon. The AraC protein is constitutively expressed and binds to the arabinose operon in the absence of the arabinose sugar. When arabinose is present, or an isomer thereof, it induces a conformational change in AraC thus preventing it from binding the operon and thus allowing transcription. Since IPTG is an isomer of β-galactosidase, it will induce the same conformational effect on AraC thus inducing transcription. In this way, the exogenous addition of IPTG will serve as a proxy for the HPV virus and induce the transcription cassette 1. Furthermore, as illustrated by Lutz and Bujard, the degree of induction is influenced by both IPTG and arabinose. Hence, promoter activity can be regulated and finely tuned by the addition of varying concentrations of IPTG and arabinose. The Lac/AraC promoter will be synthesized by primer-primer annealing and subsequent PCR elongation.
PlcR-PapR Quorum Molecule
The PlcR regulon in Bacillus thuringiensis and Bacillus cereus houses approximately 15 genes required for the production of many virulence factors. The activation of this regulon is under the control of the PlcR peptide which binds a region within the promoter known as the PlcR box. The papR gene - which is found within the PlcR regulon - encodes a 48 amino acid peptide that is crucial for the binding to and subsequent activation of the PlcR box. The PapR pro-peptide is secreted from the cell (via the SecA pathway) following translation, cleaved extra-cellularly and the resulting pentapeptide is re-imported into the cell via an oligopeptide permease (a protease ubiquitous to the extra-cellular matrix of Gram-positive bacteria). Once inside the cell, PapR allows PlcR to bind to the PlcR box thus activating the regulon. Due to the presence of the plcR gene within the regulon, PlcR positively regulates its own transcription. The PlcR regulon within Bacillus anthracis houses a gene that codes for a PlcR-PapR fusion protein which was found to strongly induce transcription of the native plcR gene. It has been shown, by Pomerantsev and colleagues, that binding to the PlcR box and activation of the PlcR regulon is achieved by the expression of a hetrologous PlcR-PapR fusion protein. So as to ectopically express both PlcR and PapR in Lactobacillus gasseri, a sequence coding for the PlcR-PapR fusion protein will be derived from bioinformatic analysis of the native sequence in Bacillus anthracis. This will be implemented due to the inherent compactness of the fusion protein as well as ease of transportability when inserting into a foreign bacteria. The plcR-papR sequence will be synthesized by Gene Art and furthermore, the sequence will be optimized by an online tool.
Venus
So as to assess the degree of activation of cassette 1, a variant of the Yellow Fluorescent Protein, Venus, has been included in the construct. So as to obtain quantitative data as to the efficacy of IPTG in inducing the activation of cassette 1, a host of fluorometric analysis will be performed on a cassette-containing colony. Venus, which possesses a known excitation:emission characteristic, will be used as a reporter protein to quantify the degree of transcription (by proxy of fluorescence) with use of a fluorometer. Although venus is a ubiquitous fluorescent protein found in the laboratory, specialised sequences will be ordered from Gene Art. Furthermore, the sequences will be codon optimized so as to produce the greatest protein yield and fluorometric activity in Lactobacillus gasseri.
Terminator
So as to compartmentalize the cassette and explicitly terminate transcription, the inclusion of a double terminator from the Registry of Standard Parts is included in the design. The terminator used to ‘cap’ cassette 1 is a rho-independent terminator and will inhibit polymerase functioning by the formation of a stem-loop structure. The terminator DNA will be sourced from the Standard Registry of Parts.
Cassette 2 in population 2
PlcR Promoter
Through the identification of the PlcR box within the promoter of the PlcR regulon, a promoter that contains the PlcR box as well the requisite transcription-initiating landmarks within the promoter will be synthesized. Hence, any construct that is placed downstream of the promoter will be activated by the addition of exogenous PlcR and the facilitating peptide PapR. The PlcR-PapR promoter will be synthesized through primer-primer annealing and subsequent PCR elongation.
PlcR-PapR Fusion Gene
The PlcR-PapR fusion gene that codes for the activating peptides PlcR and PapR has been included in construct so as to amplify the initial signal presented by population 1. Since the fusion protein of PlcR and PapR is translated into its inactive form, there will be no direct self stimulatory effects within the same bacterium. Once secreted and processed, the peptides will serve to activate neighboring bacteria thus ensuring the propagation of the signal throughout the entire population.
Phage Activator
Developed by the winners of 2009 iGEM competition, Team Cambridge developed an array of activator and promoter combinations (derived from bacteriophages) that have varying degrees of binding and activating strengths. The intention was to create a library of activators and promoters than can be combined in such a way so as to vary the degree of negative feedback SpooA exerts on the PlcR-PapR promoter. As such, several alternative endings of cassette 2 will be created, and based on experimental procedure a combination will be chosen so as to introduce an effective delay in repression. This delay is crucial so as to allow sufficient transcription of both the PlcR-PapR fusion peptide as well as the E. chromi pigment as this ensures adequate propagation of the signal throughout the colony as well as sufficient E. chromi being produced to ensure a distinctly visible report. The phage activator will be sourced from the Registry of Standard Parts.
mCherry
Akin to the function of Venus in cassette 1, mCherry - a monomeric fluorescent protein - will be used in assaying the degree of transcription induced in colony 2 by incident PlcR-PapR quorum molecules. In addition to the visual report that will be produced as the response signal that propagates throughout the colony, mCherry will aid in the quantification of the mean fluorescent response of the colony. mCherry will be sourced from the Registry of Standard Parts.
Terminator
As described above, the terminator ends transcription. By placing a terminator in the middle of cassette 2, it introduces a time delay between the activation of the initial region of cassette 2 and the feedback component, SpooA. The terminator DNA will be sourced from the Registry of Standard Parts.
PO Phage Promoter
Forming the receptor of the cognate activator-promoter pair of phage sensitivity tuners, the PO promoter will initiate transcription when the phage activator binds the promoter. Since the genes downstream of the promoter will only be transcribed after the phage activator is transcribed, translated and folded, a time delay is inserted due to this unavoidable metabolic process. The PO promoter will be sourced from the Registry of Standard Parts.
E. chromi
So as to produce a chromogenic indication of the presence of HPV (or a proxy thereof), the E. chromi pigment will be used. The E. chromi biobrick is a composite biobrick comprised of sequential enzymes that process a substrate until a visible dye is produced. The combination of four sequential enzymes converts the substrate farnesyl pyrophosphate (which is colourless) to the pigment beta-carotine via several intermediates. The E. chromi biobrick will be sourced from the Registry of Standard Parts.
SpooA
So as to provide a suitable negative feedback loop to the ‘machine’, SpooA is a protein that binds to the regions adjacent to the PlcR box in the PlcR-PapR promoter. Once produced, the repression of SpooA will negate the production of both mCherry as well as E. chromi. Furthermore, SpooA will inhbit it’s own production this derepressing cassette 2 in such a way that the system, if left to its own devices in the presence of a suitable concentration of PlcR-PapR, will exhibit oscillatory behavior. SpooA will be synthesized by Gene Art and will be codon optimized for Lactobacillus gasseri so as to ensure optimal production and expression.
Terminator
So as to provide a final cap to cassette 2 and the machine in whole, a terminator will be placed to end transcription. The terminator will be sourced from the Registry of Standard Parts.
 "
"