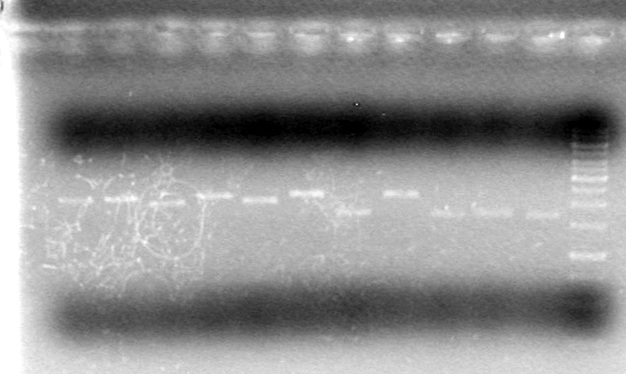
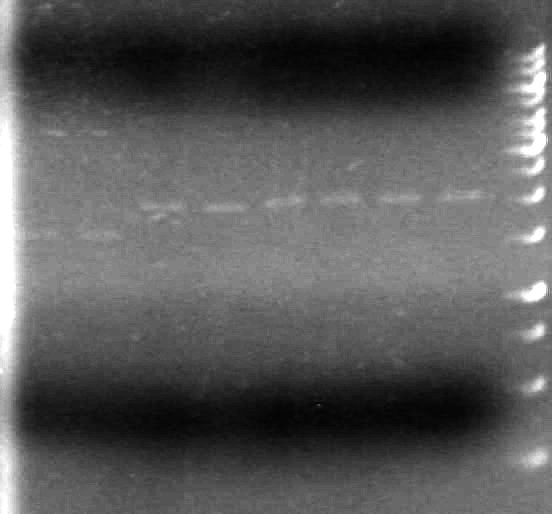
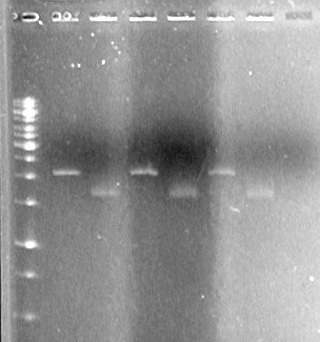
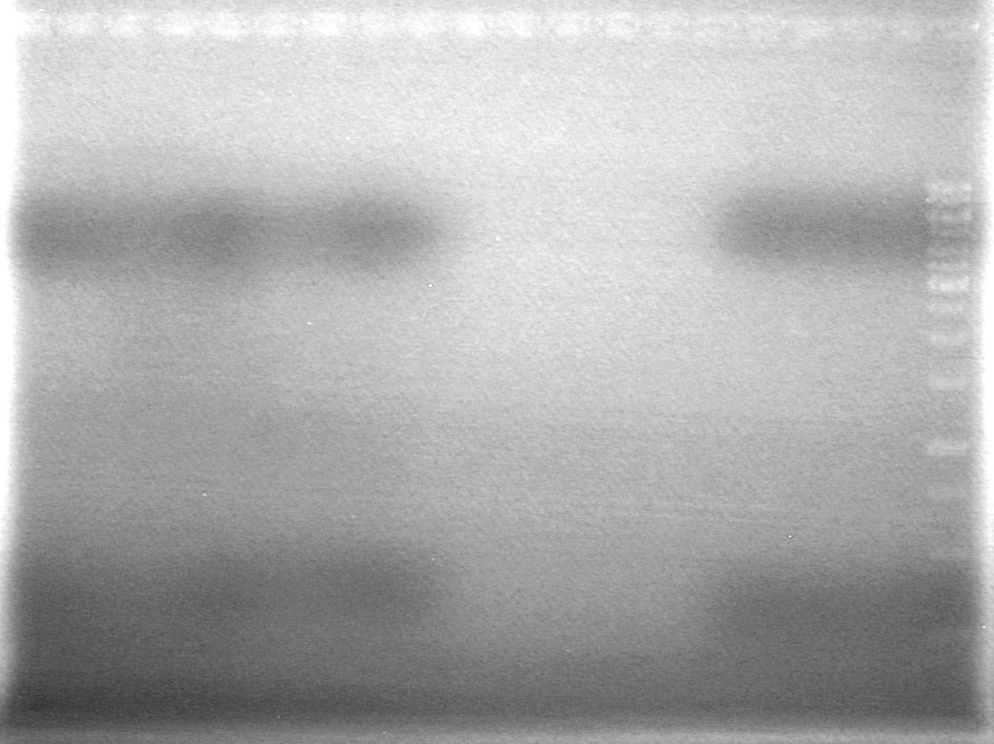
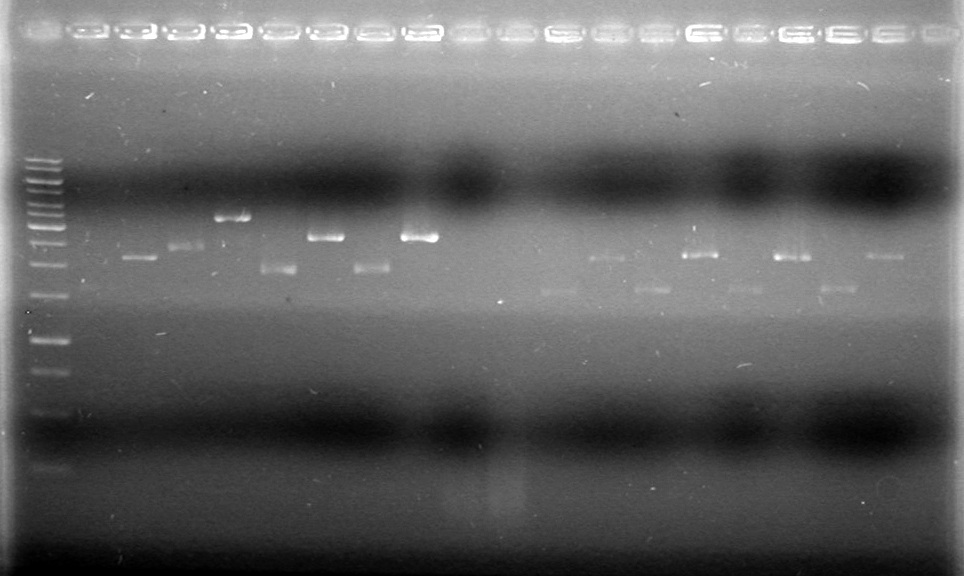
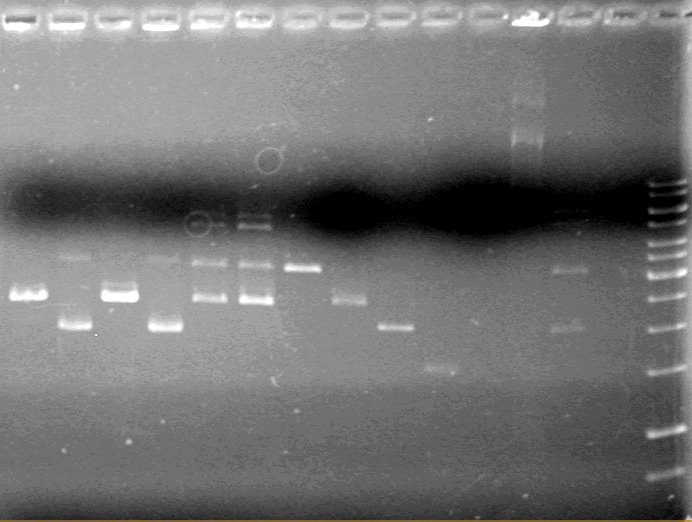
| Lane | Sample | Volume Rxn (µL) | Volume Dye (µL) |
| 1 | 1kb Ladder† | 2 | 2 |
| 2 | Restricted Fusion CEYFP (J5) | 10 | 10 |
| 3 | Unrestriced Fusion CEYFP | 10 | 10 |
| 4 | Restricted ECFP (B3) | 10 | 10 |
| 5 | Unrestricted ECFP (B3) | 10 | 10 |
| 6 | Restricted EYFP (B6) | 10 | 10 |
| 7 | Unrestricted EYFP (B6) | 10 | 10 |
| 8 | Restricted NEYFP (I6) | 10 | 10 |
| 9 | Unrestricted NEYFP (I6) | 10 | 10 |
| 10 | Restricted EYFP (A6) | 10 | 10 |
| 11 | Unrestricted EYFP (A6) | 10 | 10 |
| 12 | Restricted EYFP (J6) | 10 | 10 |
| 13 | Unrestricted EYFP (J6) | 10 | 10 |
† Also added 8µL of water to this mix
Ran both gels at 100V for 2 hours.
Results:
Gel 1
Gel 2
Conclusion: To be concluded....
Objective: Transform DH5α cells with plasmids containing ligation products from May 25/2010.
Method:
Transform, using the competent cell transformation protocol, the ligation products with the most intense banding from each of the following ligation reactions:
- mms6 + dT (B6 + A6)
- xylE + dT (B4 + C4)
Incubate at 37oC overnight.
Results:
No colonies grew, even on positive control plate.
May 31/2010
Objective: Transform part BBa_J33204 (Ribosomal Binding Site + xylE gene) into DH5α cells.
Method:
Obtained part BBa_J33204 from 2010 iGEM Spring Distribution Kit Plate 1, well 7P.
Transformed using competent cell transformation protocol, along with negative control (milliQ H2O water) and positive control (pUC19 plasmid).
Plated 100µL and 50µL on separate pre-warmed LB agar plates containing 100µg/mL ampicillin.
Added 250µL of SOC media and incubated for 90 minutes at 37o.
Results:
Obtained the following number of colonies on each plate:
- BBa_J33204 50µL - 13 colonies
- BBa_J33204 100µL - 18 colonies
- pUC19 - 9 colonies
Other lab activities:
- Inoculated 5mL of LB liquid broth (Amp+ with cells containing Bba_J33204 picked from colony off transformation plate and incubated at 37oC with shaking overnight.
AV quantified each pDNA stock in the working plasmids box by measuring the A260 of a 1:100 dilution of the pDNA samples in a UV cuvette. Results are posted on the working plasmids page.
May 31/2010 - Evening
Objective: Transform recent ligation reactions that didn't work the first time around.
Method: Use competent cell transformation protocol to transform the following ligation products:
- pLacI + sRBS
- pLacI + sRBS-Lum-dT (times 2)
- mms6 (B6) + dT
- mms6 (A6) + dT
- xylE (C4) + dT
- xylE (B4) + dT
Plated all cells; spun down media so cells were pelleted and removed 200uL of cell-free media. Re-suspended pelleted cells in remaining media, and plated onto ampicillin plates.
Results:
No growth on plates EXCEPT:
- Positive control (pUC19) - 9 colonies
- mms6 (B6) + dT - 1 colonies
 "
"