|
SEPTEMBER: WEEK 3
September, 13th
Unfortunately we discovered we tried to ligate I32 (E-S) to I37 (X-P) instead of I37 (E-X) ;-(
So this step will be repeated.
Trasformation of ligations
into E. coli DH5-alpha.
Inoculum of
into 5 ml LB+Amp for ligations of the following day.
Inoculum from single colony of MG42 and MC43 in 5 ml LB+Cm12.5, than ON at 37°C.
September, 14th
Finally Mr.Gene sent us our YEAST: a little difficult to pick up the package but at the end we succeeded!
I52, I54, I55, I56, I57, I58 plates showed in general few colonies; I52 and I5X showed very few colonies (<=5). Very strange (mumble mumble...), they will be screened ASAP (stored at +4°C).
Miniprep and quanfification of:
- I26: 69,7 ng/ul
- I31: 89,5 ng/ul
- I34: 147,6 ng/ul
3-hours digestion:
- I26: XbaI-PstI (Insert)
- I31: EcoRI-SpeI (Insert)
- I34: E-X (Vector)
Gel run/cut of samples (I26 and I31 insert bands were very very soft)
 Gel run/cut for digested I26, I31 and I34. and gel extraction:
- I26 (X-P): 2,6 ng/ul
- I31 (E-S): 1,4 ng/ul
- I34 (E-X): ng/ul
We already had digested DNA so we could perform new ON ligations
- I59: I21 (E-S) + I34 (E-X)
- I60: I20 (S-P) + I26 (X-P)
- I61: I31 (E-S) + I37 (E-X)
and repeat
- I53: I32 (E-S) + I37 (E-X)
Inoculum of I47, I48, I49 into 5 ml LB+Amp for TECAN test of the following day.
Screening PCR on 3 colonies picked from MC42 and MC43 respectively.
Two method were used: our standard PCR picking a colony from the plate and using it for the PCR and a different protocol picking the colony and treating it with a 95°C 5 min step before the PCR.
The results were the follow:
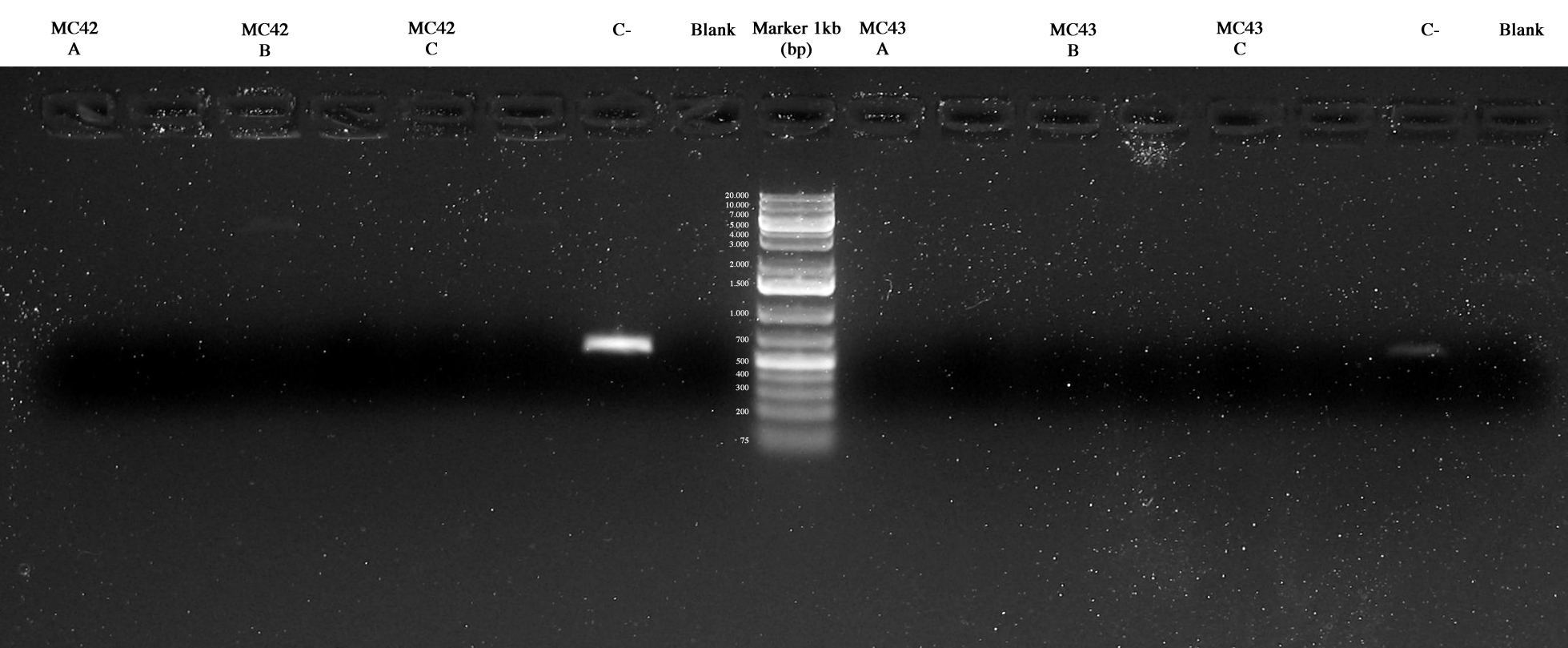 MC42A/B/C, Cneg, blank, MC43A/B/C, Cneg, blank. Negative controls were ok and maybe MC42_C and MC43_C were ok too but further investigations were necessary.
Tecan Test was performed on prepared samples, after the usual protocol (dilution, medium change and dilution 1:1000).
September, 15th
I47, I48, I49 cultures were diluted 1:100 into 5ml LB+Amp.
In the afternoon all cultures were synchronized to O.D. 0,02 and Tecan Test was started to check GFP production by these BioBricks.
Transformation of I 53, I59, I60, I61 ligations into E. coli DH5-alpha. They were plated on LB+Amp agar plates and let grow ON at 37°C.
September, 16th
All plates showed colonies, so we could perform screening through "colony PCR" for ligations:
- I52
- I53
- I54
- I55
- I56
- I57
- I58
- I59
- I60
- I61
For each plate we picked X colonies that were also inoculated into 1 ml LB+Amp in order to be ready to make glycerol stock of positive ones.
Agarose gel was prepared and samples loaded and run:
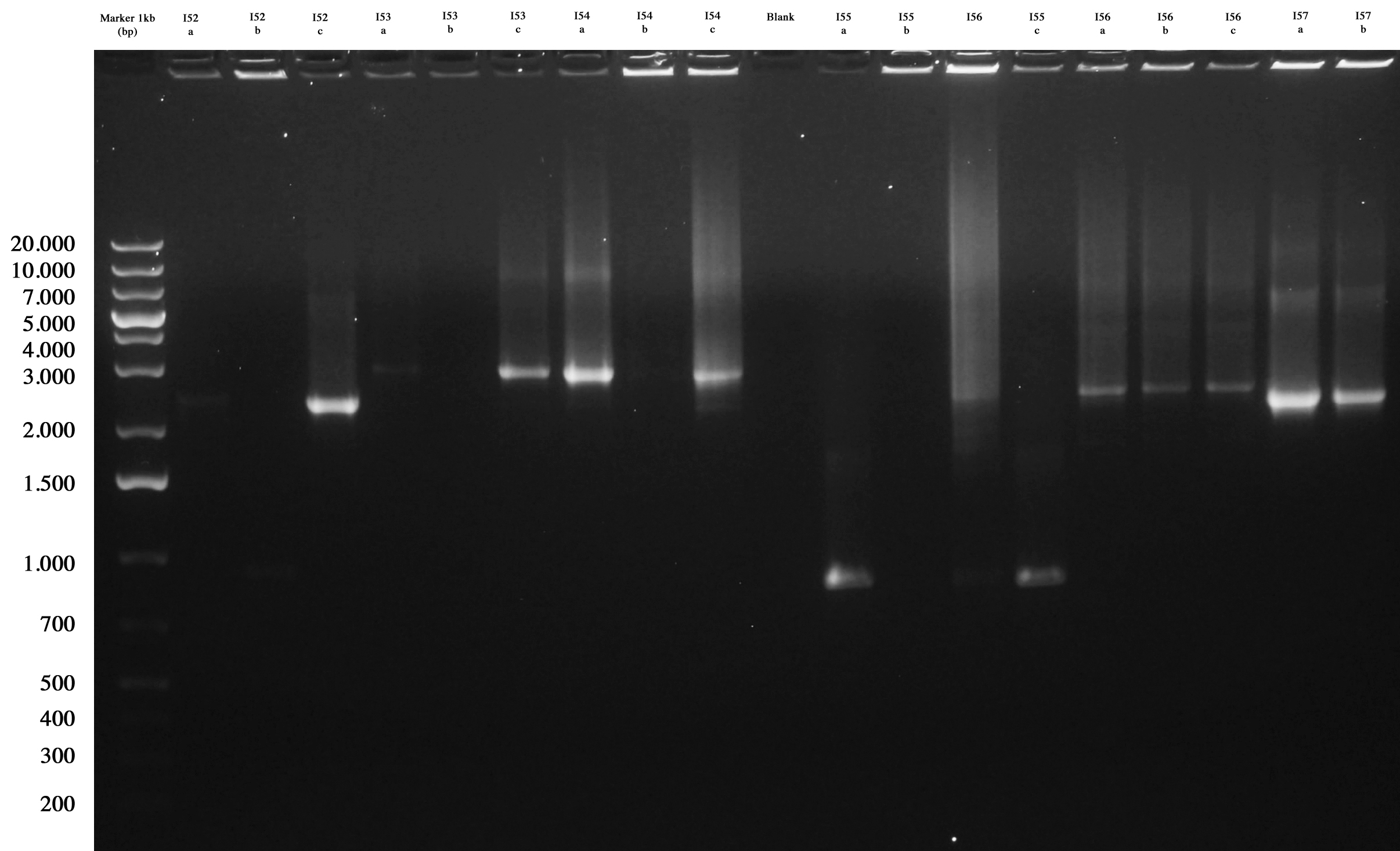 Gel run for ligations amplified through PCR. As you can see ...
Tecan Test was performed on prepared samples, after the usual protocol (dilution, medium change and dilution 1:1000).
September, 17th
September, 18th
|
 "
"



