From 2010.igem.org
(Difference between revisions)
|
|
| Line 1,460: |
Line 1,460: |
| | <br> | | <br> |
| | | | |
| - | ==9/6 ~ 9/9 PCR== | + | ==September 6 ~ Septembr 9== |
| | | | |
| | <span style=font-size:12px><b>PCR composition </b></span> | | <span style=font-size:12px><b>PCR composition </b></span> |
Revision as of 16:46, 12 September 2010
September 1
pREP41 vector has arrived.
-> we will do E.coli transformation and increase its number.
pREP42-GFP vector has arrived (in E.coli)
-> will incubate a day and do spreading for further experiment.
Making culture media
- 500mL LB broth + agar 1.5% (7.5g)
- Do auto clave
- Cool it down until it reaches 50~60℃
- Add ampicillin(1000x 100mg/mL) and stir
- putting out bubbles on culture media
- pouling on petri dish and wait for 1 hour
Culture
- Competent cell + DN10A and leave it in ice for 30min
- Do heat shock for 45 sec and store it in ice for 2min
- Stabilize it in incubator for 30 to 40min
- Do spreading
September 2 ~ September 5
PCR Setting
V_STAT1 (expression)

| Primer | Direction | Sequences | Length |
| V_STAT_F | Forward(NdeI) | 5’-TGTTCATATGGCTAATGTCTCAGTGGTACGAACTTCAG-3’ | 24bp |
| V_STAT_R | Reverse(BamHI) | 5’-TATCGGATCCGAATTTACACTTCAGACACAGAAATCAAC-3’ | 25bp |
| Materials | Amount |
| STAT1(KRIBB) (template) | 150pmole |
| V_STAT_F | 50pmole |
| V_STAT_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

STAT1 (submission)

| Primer | Direction | Sequences | Length |
| STAT_F | Forward | 5’-ATGTCTCAGTGGTACGAACTTCAG-3’ | 24bp |
| STAT_R | Reverse | 5’-TTACACTTCAGACACAGAAATCAAC-3’ | 25bp |
| Materials | Amount |
| STAT1(KRIBB) (template) | 150pmole |
| STAT_F | 50pmole |
| STAT_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

Fusion Antibody Receptor (Submission)

| Primer | Direction | Sequences | Length |
| SIG_F | Forward | 5’-ATGGTCTTTTTAAATTCCTCTCCC-3’ | 24bp |
| Ab_R | Reverse | 5’-GGGGCTGTTGTTTTGGCTGAGG-3’ | 22bp |
| Materials | Amount |
| Fusion antibody receptor T vector (template) | 150pmole |
| SIG_F | 50pmole |
| Ab_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

Signal Peptide (Submission)

| Primer | Direction | Sequences | Length |
| SIG_F | Forward | 5’-ATGGTCTTTTTAAATTCCTCTCCC-3’ | 24bp |
| SIG_R | Reverse | 5’-AGCCGCCACCAACCGAGTAGAAA-3’ | 23bp |
| Materials | Amount |
| Fusion antibody receptor T vector (template) | 150pmole |
| SIG_F | 50pmole |
| SIG_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

Ig-like (Submission)

| Primer | Direction | Sequences | Length |
| IG_F | Forward | 5’-AGGCCGTCCCCGACCTTGCCTG-3’ | 22bp |
| IG_R | Reverse | 5’-GGAGTCATCATCATCATCATCATC-3’ | 24bp |
| Materials | Amount |
| Fusion antibody receptor T vector (template) | 150pmole |
| IG_F | 50pmole |
| IG_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

Signal Peptide + Ig-like (Submission)

| Primer | Direction | Sequences | Length |
| SIG_F | Forward | 5’-ATGGTCTTTTTAAATTCCTCTCCC-3’ | 24bp |
| IG_R | Reverse | 5’-GGAGTCATCATCATCATCATCATC-3’ | 24bp |
| Materials | Amount |
| Fusion antibody receptor T vector (template) | 150pmole |
| SIG_F | 50pmole |
| IG_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

Antibody (Submission)

| Primer | Direction | Sequences | Length |
| Ab_F | Forward | 5’-ACCCAGTCTCCAGCAATCATGTC-3’ | 23bp |
| Ab_R | Reverse | 5’-GGGGCTGTTGTTTTGGCTGAGG-3’ | 22bp |
| Materials | Amount |
| Fusion antibody receptor T vector (template) | 150pmole |
| Ab_F | 50pmole |
| Ab_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

V_Fusion Antibody Receptor (Expression)

| Primer | Direction | Sequences | Length |
| SIG_F | Forward | 5’-ATGGTCTTTTTAAATTCCTCTCCC-3’ | 24bp |
| Linker_R | Reverse | 5’-CTCTCTTCCAGGGCTTCCAGAACGGGGCTGTTGTTTTGGCTGAGGA-3’ | 46(23+23)bp |
| Materials | Amount |
| Fusion antibody receptor T vector (template) | 150pmole |
| SIG_F | 50pmole |
| Linker_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

B_FGFR1 (Submission)

| Primer | Direction | Sequences | Length |
| B_FGFR_F | Forward | 5’-GTTCTGGAAGCCCTGGAAGAGAG-3’ | 23bp |
| B_FGFR_R | Reverse | 5’-TCAGCGGCGTTTGAGTCCGCCAT-3’ | 23bp |
| Materials | Amount |
| FGFR1(KRIBB) (template) | 150pmole |
| B_FGFR_F | 50pmole |
| B_FGFR_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

VB_FGFR1 (Expression)

| Primer | Direction | Sequences | Length |
| Linker_F | Forward | 5’-TCCTCAGCCAAAACAACAGCCCCGTTCTGGAAGCCCTGGAAGAGAG-3’ | 46(23+23)bp |
| B_FGFR_R | Reverse | 5’-TCAGCGGCGTTTGAGTCCGCCAT-3’ | 23bp |
| Materials | Amount |
| FGFR1(KRIBB) (template) | 150pmole |
| Linker_F | 50pmole |
| B_FGFR_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

Fusion Antibody Receptor (Expression)

| Primer | Direction | Sequences | Length |
| SIG_F | Forward | 5’-ATGGTCTTTTTAAATTCCTCTCCC-3’ | 24bp |
| B_FGFR_R | Reverse | 5’-TCAGCGGCGTTTGAGTCCGCCAT-3’ | 23bp |
| Materials | Amount |
| V_Fusion antibody receptor (template) | 150pmole |
| VB_FGFR1(2+3) (template) | 150pmole |
| SIG_F | 50pmole |
| B_FGFR_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

5’UTR (expression)
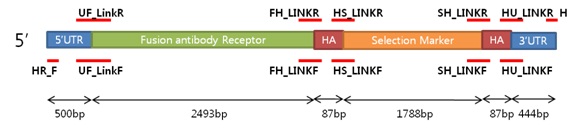
| Primer | Direction | Sequences | Length |
| HR_F | Forward | 5’-AAACCAGTTTTGCCAAAACAAC-3’ | 22bp |
| UF_LinkR | Reverse | 5’ –GAGAGGAATTTAAAAAGACCATAAACAACATTTTTCTTTTTTAC - 3’ | 44(22+22)bp |
| Materials | Amount |
| S.pombe genomic DNA (template) | 150pmole |
| HR_F | 50pmole |
| UF_LinkR | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

3’UTR (expression)

| Primer | Direction | Sequences | Length |
| HU_LINKF (HA2 + 3’UTR) | Forward | 5’-CCATATGACCCAGATTACGCTTGGAAATTAAATCGTGCGTAAG-3’ | 43(21+22)bp |
| HR_R | Reverse | 5’ – GCGTTCTTTTTTAATTCTGAATTAA – 3’ | 25bp |
| Materials | Amount |
| S.pombe genomic DNA (template) | 150pmole |
| HU_LINKF (HA2 + 3’UTR) | 50pmole |
| HR_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

Selection Marker (expression)

| Primer | Direction | Sequences | Length |
| HS_LINKF (HA1 + Selection Marker) | Forward | 5’-CCATATGACCCAGATTACGCTAGCAAGCTTAAGCTTAGCTAC-3’ | 42(21+21)bp |
| SH_LINKR | Reverse | 5’-GTCAGGAACATCGTATGGGTAGACCTGCAGAAGCTTAAGCTTG-3’ | 43(21+22)bp |
| Materials | Amount |
| pREP42-GFP vector (template) | 150pmole |
| HS_LINKF (HA1 + Selection Marker) | 50pmole |
| SH_LINKR | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

Core promoter + GFP (expression)

| Primer | Direction | Sequences | Length |
| PC_LINKF (Proximal + Core) | Forward | 5’–CAGAATAGACACACGGGCCGACATTGAAGATATATAAAGGAAG–3’ | 43(20+23)bp |
| CGFP_R (Core promoter + GFP) | Reverse | 3’-CGACATATGCTTGTACAGCTCG-5’ | 22bp |
| Materials | Amount |
| pREP42-GFP vector (template) | 150pmole |
| PC_LINKF (Proximal + Core) | 50pmole |
| CGFP_R (Core promoter + GFP) | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

Proximal promoter (expression)

| Primer | Direction | Sequences | Length |
| PP_F (Proximal promoter) | Forward | 5’-CGCCTGCAGGCGAATAGCTGG–3’ | 21bp |
| PC_LINKR | Reverse | 5’–CTTCCTTTATATATCTTCAATGTCGGCCCGTGTGTCTATTCTG–3’ | 43(23+20)bp |
| Materials | Amount |
| Proximal promoter T vector (template) | 150pmole |
| PP_F (Proximal promoter) | 50pmole |
| PC_LINKR | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

HA1 (expression)

| Primer | Direction | Sequences | Length |
| FH_LINKF (FGFR + HA1) | Forward | 5’-GCGGACTCAAACGCCGCTGATACCCATACGATGTTCCTGAC-3’ | 41(20+21)bp |
| HS_LINKR | Reverse | 5’-GTAGCTAAGCTTAAGCTTGCTAGCGTAATCTGGGTCATATGG-3’ | 42(21+21)bp |
| Materials | Amount |
| HA T vector (template) | 150pmole |
| FH_LINKF (FGFR + HA1) | 50pmole |
| HS_LINKR | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

HA2 (expression)

| Primer | Direction | Sequences | Length |
| SH_LINKF (Selection Marker + HA2) | Forward | 5’-CAAGCTTAAGCTTCTGCAGGTCTACCCATACGATGTTCCTGAC-3’ | 43(22+21)bp |
| HU_LINKR | Reverse | 5’-CTTACGCACGATTTAATTTCCAAGCGTAATCTGGGTCATATGG-3’ | 43(22+21)bp |
| Materials | Amount |
| HA T vector (template) | 150pmole |
| SH_LINKF (Selection Marker + HA2) | 50pmole |
| HU_LINKR | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

Fusion antibody receptor (expression)

| Primer | Direction | Sequences | Length |
| UF_LinkF (5’UTR+FGFR Linker) | Forward | 5’-GTAAAAAAGAAAAATGTTGTTTATGGTCTTTTTAAATTCCTCTC-3’ | 44(22+22)bp |
| FH_LINKR | Reverse | 5’-GTCAGGAACATCGTATGGGTATCAGCGGCGTTTGAGTCCGC-3’ | 41(21+20)bp |
| Materials | Amount |
| Fusion Antibody receptor (template) | 150pmole |
| UF_LinkF (5’UTR+FGFR Linker) | 50pmole |
| FH_LINKR | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

5’UTR + Fusion Antibody receptor (expression)

| Primer | Direction | Sequences | Length |
| HR_F (for homologous recombinant) | Forward | 5’-AAACCAGTTTTGCCAAAACAAC-3’ | 22bp |
| FH_LINKR | Reverse | 5’-GTCAGGAACATCGTATGGGTATCAGCGGCGTTTGAGTCCGC-3’ | 41(21+20)bp |
| Materials | Amount |
| 5'UTR (template) | 150pmole |
| Fusion Antibody receptor (template) | 150pmole |
| FH_LINKF (FGFR + HA1) | 50pmole |
| SH_LINKR | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

HA1 + Selection Marker (expression)

| Primer | Direction | Sequences | Length |
| FH_LINKF (FGFR + HA1) | Forward | 5’-GCGGACTCAAACGCCGCTGATACCCATACGATGTTCCTGAC-3’ | 41(20+21)bp |
| SH_LINKR | Reverse | 5’-GTCAGGAACATCGTATGGGTAGACCTGCAGAAGCTTAAGCTTG-3’ | 43(21+22)bp |
| Materials | Amount |
| HA1 (template) | 150pmole |
| Selection Marker (template) | 150pmole |
| FH_LINKF (FGFR + HA1) | 50pmole |
| SH_LINKR | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

HA2 + 3'UTR (expression)

| Primer | Direction | Sequences | Length |
| SH_LINKF (Selection Marker + HA2) | Forward | 5’-CAAGCTTAAGCTTCTGCAGGTCTACCCATACGATGTTCCTGAC-3’ | 43(22+21)bp |
| HR_R | Reverse | 5’ – GCGTTCTTTTTTAATTCTGAATTAA – 3’ | 25bp |
| Materials | Amount |
| HA2 (template) | 150pmole |
| 3’UTR (template) | 150pmole |
| SH_LINKF (Selection Marker + HA2) | 50pmole |
| HR_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

5’UTR - Fusion Antibody receptor + HA1 - Selection Marker (expression)

| Primer | Direction | Sequences | Length |
| HR_F(for homologous recombinant) | Forward | 5’-AAACCAGTTTTGCCAAAACAAC-3’ | 22bp |
| SH_LINKR | Reverse | 5’-GTCAGGAACATCGTATGGGTAGACCTGCAGAAGCTTAAGCTTG-3’ | 43(21+22)bp |
| Materials | Amount |
| 5’UTR - Fusion Antibody receptor (template)
| 150pmole |
| HA1 - Selection Marker (template)
| 150pmole |
| HR_F (for homologous recombinant) | 50pmole |
| SH_LINKR | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

Homologous Recombinant (expression)

| Primer | Direction | Sequences | Length |
| HR_F(for homologous recombinant) | Forward | 5’-AAACCAGTTTTGCCAAAACAAC-3’ | 22bp |
| HR_R | Reverse | 5’ – GCGTTCTTTTTTAATTCTGAATTAA – 3’ | 25bp |
| Materials | Amount |
| HA2 + 3'UTR (template)
| 150pmole |
| 5’UTR - Fusion Antibody receptor - HA1 - Selection Marker (template)
| 150pmole |
| HR_F (for homologous recombinant) | 50pmole |
| HR_R | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |

September 6 ~ Septembr 9
PCR composition
| Materials | Amount |
| Template | 100pmole |
| Primer A | 50pmole |
| Primer B | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 2ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |
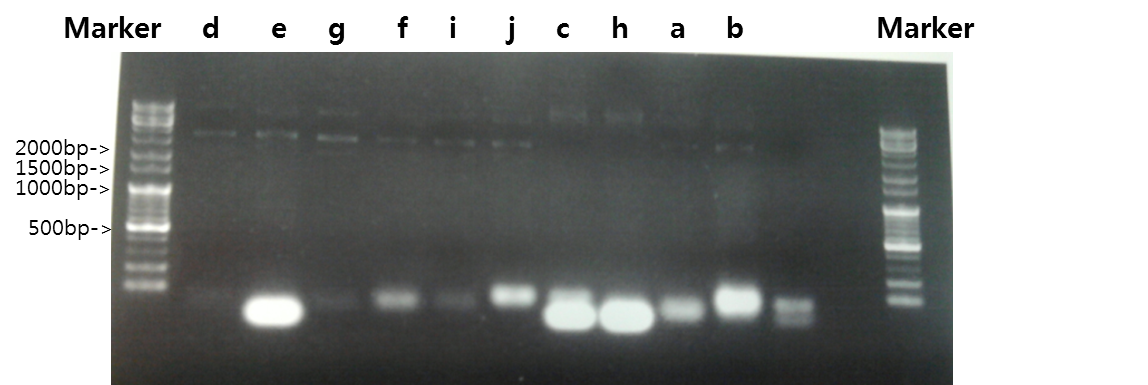
Exp 1
| Segment | Annealing Temperature | Result |
| a | STAT1 | 53*C | - | - |
| b | V_STAT1 | 53*C | - | - |
| c | Fusion Antibody receptor | 53*C | - | - |
| d | Signal peptide | 56*C | - | - |
| e | Ig-like | 56*C | - | - |
| f | Signal Peptide + Ig-like | 56*C | - | - |
| g | Antibody | 56*C | - | - |
| h | V_Fusion Antibody Receptor | 53*C | - | - |
| i | B_FGFR1 | 56*C | - | - |
| j | VB_FGFR1 | 56*C | - | - |
Exp 2
| Segment | Annealing Temperature | Result |
| a | STAT1 | 48*C | 60% | 2139bp |
| b | V_STAT1 | 48*C | - | - |
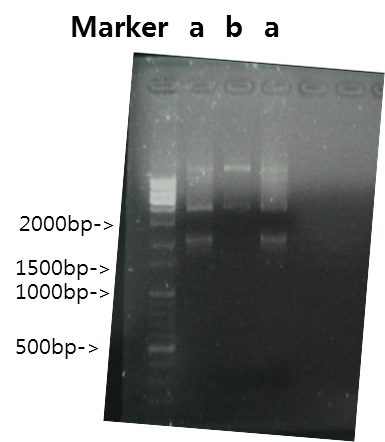
Exp 3
| Segment | Annealing Temperature | Result |
| a | STAT1 | 50*C | 100% | 2139bp |
| b | V_STAT1 | 50*C | - | - |
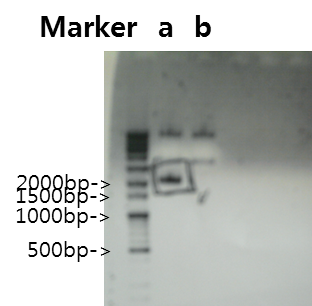
Exp 4
| Segment | Annealing Temperature | Result |
| a | Fusion Antibody receptor | 50*C | - | - |
| b | Signal peptide | 50*C | - | - |
| c | Ig-like | 50*C | 60% | 339bp |
| d | Signal Peptide + Ig-like | 50*C | - | - |
| e | Antibody | 50*C | - | - |
| f | V_Fusion Antibody Receptor | 50*C | - | - |

9/10 ~ 9/12 PCR
PCR composition
| Materials | Amount |
| Template | 150pmole |
| Primer A | 50pmole |
| Primer B | 50pmole |
| dNTP (dNTP mixture 2.5mM TaKaRa) | 5ul |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | 0.25ul |
| 10x buffer (10x cloned Pfu Reaction buffer) | 5ul |
| DW (3rd sterile water) | rest |
| Total | 50ul |
Exp 1
| Segment | Annealing Temperature | Result |
| a | STAT1 | 60*C | - | - |
| b | V_STAT1 | 60*C | - | - |
| c | Fusion Antibody receptor | 60*C | 70% | 1095bp |
| d | Signal peptide | 60*C | - | - |
| e | Ig-like | 60*C | 60% | 339bp |
| f | Signal Peptide + Ig-like | 60*C | - | - |
| g | Antibody | 60*C | 100% | 669bp |
| h | V_Fusion Antibody Receptor | 60*C | - | - |
| i | B_FGFR1 | 60*C | - | - |
| j | VB_FGFR1 | 60*C | - | - |
| x1 | negative 1 | 60*C | - | - |
| x2 | negative 2 | 60*C | - | - |
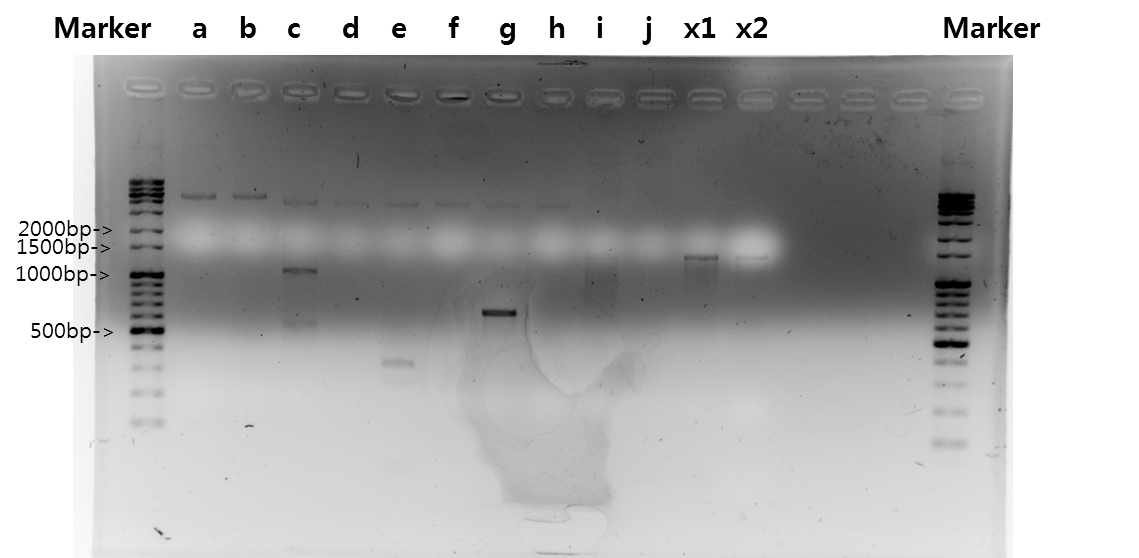
Exp 2
| Segment | Annealing Temperature | Result |
| a | STAT1 | 58*C | 60% | 2139bp |
| b | V_STAT1 | 58*C | - | - |
| c | Fusion Antibody receptor | 58*C | 80% | 1095bp |
| d | Signal peptide | 58*C | 80% | 87bp |
| e | Ig-like | 58*C | 100% | 339bp |
| f | Signal Peptide + Ig-like | 58*C | 80% | 426bp |
| g | Antibody | 58*C | 100% | 669bp |
| h | V_Fusion Antibody Receptor | 58*C | - | - |
| i | B_FGFR1 | 58*C | - | - |
| j | VB_FGFR1 | 58*C | - | - |
| x | negative | 58*C | - | - |
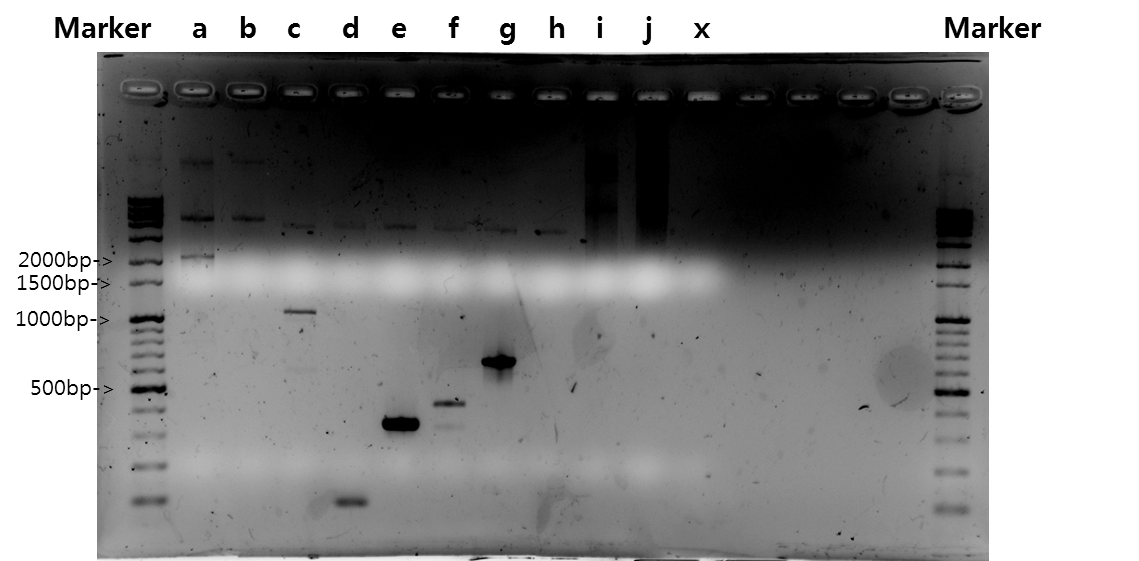
Exp 3
| Segment | Annealing Temperature | Result |
| a | STAT1 | 62*C | - | - |
| b | V_STAT1 | 62*C | - | - |
| c | Fusion Antibody receptor | 62*C | 10% | 1095bp |
| d | Signal peptide | 62*C | - | - |
| e | Ig-like | 62*C | - | - |
| f | Signal Peptide + Ig-like | 62*C | - | - |
| g | Antibody | 62*C | - | - |
| h | V_Fusion Antibody Receptor | 62*C | - | - |
| i | B_FGFR1 | 62*C | - | - |
| j | VB_FGFR1 | 62*C | - | - |
| x | negative | 62*C | - | - |

Exp 4
| Segment | Annealing Temperature | Result |
| a | STAT1 | 56*C | - | - |
| b | V_STAT1 | 56*C | - | - |
| c | Fusion Antibody receptor | 56*C | 5% | 1095bp |
| d | Signal peptide | 56*C | - | - |
| e | Ig-like | 56*C | 80% | 339bp |
| f | Signal Peptide + Ig-like | 56*C | - | - |
| g | Antibody | 56*C | 80% | 669bp |
| h | V_Fusion Antibody Receptor | 56*C | - | - |
| i | B_FGFR1 | 56*C | - | - |
| j | VB_FGFR1 | 56*C | - | - |
| x | negative | 56*C | - | - |
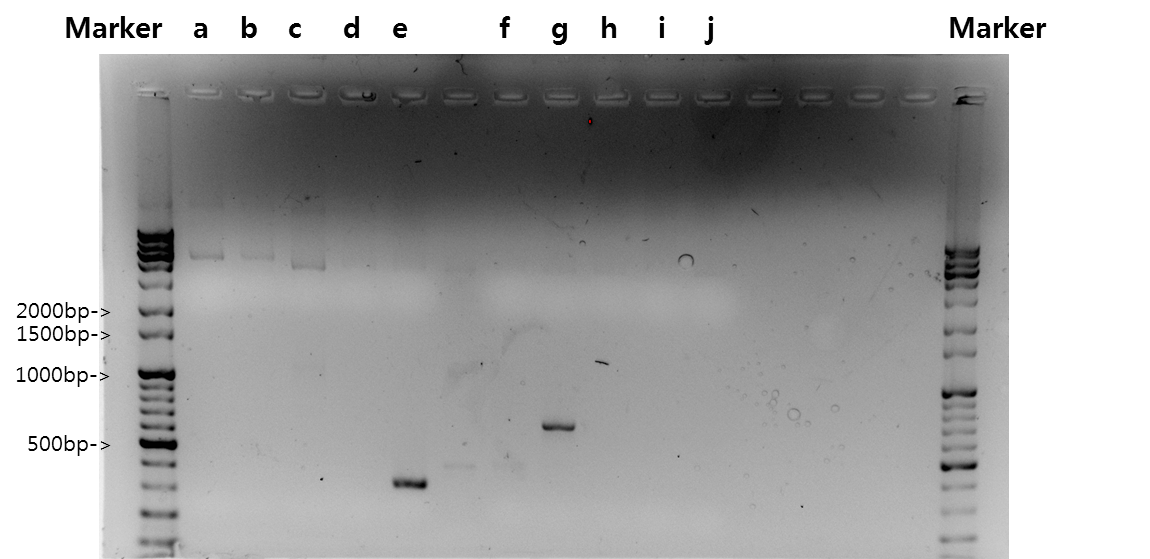
Exp 5
| Segment | Annealing Temperature | Result |
| a | V_STAT1 | 64*C | - | - |
| b | B_FGFR1 | 64*C | 5% | 1398bp |
| c | VB_FGFR1 | 64*C | - | - |
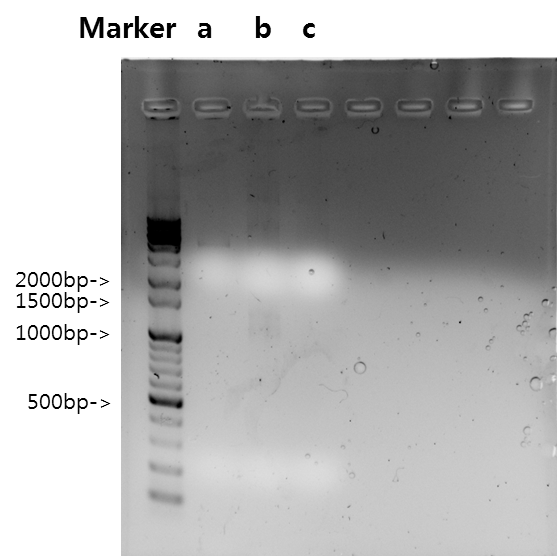
Exp 6
| Segment | Annealing Temperature | Result |
| a | V_STAT1 | 64*C | - | - |
| b | B_FGFR1 | 64*C | 3% | 1398bp |
| c | VB_FGFR1 | 64*C | - | - |

Exp 7
| Segment | Annealing Temperature | Result |
| a | 3'UTR | 62*C | - | - |
| b | 5'UTR | 62*C | - | - |
Exp 8
| Segment | Annealing Temperature | Result |
| a | V_STAT1 | 68*C | - | - |
| b | B_FGFR1 | 68*C | 10% | 1398bp |
| c | VB_FGFR1 | 68*C | - | - |

Exp 9
| Segment | Annealing Temperature | Result |
| a | V_STAT1 | 70*C | - | - |
| b | B_FGFR1 | 70*C | 3% | 1398bp |
| c | VB_FGFR1 | 70*C | - | - |
| c | 5'UTR | 70*C | - | - |
| c | 3'UTR | 70*C | - | - |

Exp 10
| Segment | Annealing Temperature | Result |
| a | V_STAT1 | 67*C | - | - |
| b | B_FGFR1 | 67*C | 6% | 1398bp |
| c | VB_FGFR1 | 67*C | - | - |
|
 "
"

























