From 2010.igem.org
(Difference between revisions)
|
|
| Line 241: |
Line 241: |
| | <table border=1> | | <table border=1> |
| | <tr align=center> | | <tr align=center> |
| - | <td>Primer</td><td>Direction</td><td>Sequences</td><td>Length</td> | + | <td><b>Primer</b></td><td><b>Direction</b></td><td><b>Sequences</b></td><td><b>Length</b></td> |
| | </tr> | | </tr> |
| | <tr align=center> | | <tr align=center> |
Revision as of 14:27, 10 September 2010
September 1
pREP41 vector has arrived.
-> we will do E.coli transformation and increase its number.
pREP42-GFP vector has arrived (in E.coli)
-> will incubate a day and do spreading for further experiment.
Making culture media
- 500mL LB broth + agar 1.5% (7.5g)
- Do auto clave
- Cool it down until it reaches 50~60℃
- Add ampicillin(1000x 100mg/mL) and stir
- putting out bubbles on culture media
- pouling on petri dish and wait for 1 hour
Culture
- Competent cell + DN10A and leave it in ice for 30min
- Do heat shock for 45 sec and store it in ice for 2min
- Stabilize it in incubator for 30 to 40min
- Do spreading
September ?
V_STAT1 (expression)

| Primer | Direction | Sequences | Length |
| V_STAT_F | Forward(NdeI) | 5’-TGTTCATATGGCTAATGTCTCAGTGGTACGAACTTCAG-3’ | 24bp |
| V_STAT_R | Reverse(BamHI) | 5’-TATCGGATCCGAATTTACACTTCAGACACAGAAATCAAC-3’ | 25bp |
| Materials | Volume |
| STAT1(KRIBB) (template) | |
| V_STAT_F | |
| V_STAT_R | |
| dNTP (dNTP mixture 2.5mM TaKaRa) | |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | |
| 10x buffer (10x cloned Pfu Reaction buffer) | |
| DW (3rd sterile water) | |
| Total | 50ul |

STAT1 (submission)

| Primer | Direction | Sequences | Length |
| STAT_F | Forward | 5’-ATGTCTCAGTGGTACGAACTTCAG-3’ | 24bp |
| STAT_R | Reverse | 5’-TTACACTTCAGACACAGAAATCAAC-3’ | 25bp |
| Materials | Volume |
| STAT1(KRIBB) (template) | |
| STAT_F | |
| STAT_R | |
| dNTP (dNTP mixture 2.5mM TaKaRa) | |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | |
| 10x buffer (10x cloned Pfu Reaction buffer) | |
| DW (3rd sterile water) | |
| Total | 50ul |

Fusion Antibody Receptor (Submission)

| Primer | Direction | Sequences | Length |
| SIG_F | Forward | 5’-ATGGTCTTTTTAAATTCCTCTCCC-3’ | 24bp |
| Ab_R | Reverse | 5’-GGGGCTGTTGTTTTGGCTGAGG-3’ | 22bp |
| Materials | Volume |
| Fusion antibody receptor T vector (template) | |
| SIG_F | |
| Ab_R | |
| dNTP (dNTP mixture 2.5mM TaKaRa) | |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | |
| 10x buffer (10x cloned Pfu Reaction buffer) | |
| DW (3rd sterile water) | |
| Total | 50ul |

Signal Peptide (Submission)

| Primer | Direction | Sequences | Length |
| SIG_F | Forward | 5’-ATGGTCTTTTTAAATTCCTCTCCC-3’ | 24bp |
| SIG_R | Reverse | 5’-AGCCGCCACCAACCGAGTAGAAA-3’ | 23bp |
| Materials | Volume |
| Fusion antibody receptor T vector (template) | |
| SIG_F | |
| SIG_R | |
| dNTP (dNTP mixture 2.5mM TaKaRa) | |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | |
| 10x buffer (10x cloned Pfu Reaction buffer) | |
| DW (3rd sterile water) | |
| Total | 50ul |

Ig-like (Submission)

| Primer | Direction | Sequences | Length |
| IG_F | Forward | 5’-AGGCCGTCCCCGACCTTGCCTG-3’ | 22bp |
| IG_R | Reverse | 5’-GGAGTCATCATCATCATCATCATC-3’ | 24bp |
| Materials | Volume |
| Fusion antibody receptor T vector (template) | |
| IG_F | |
| IG_R | |
| dNTP (dNTP mixture 2.5mM TaKaRa) | |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | |
| 10x buffer (10x cloned Pfu Reaction buffer) | |
| DW (3rd sterile water) | |
| Total | 50ul |

Signal Peptide + Ig-like (Submission)

| Primer | Direction | Sequences | Length |
| SIG_F | Forward | 5’-ATGGTCTTTTTAAATTCCTCTCCC-3’ | 24bp |
| IG_R | Reverse | 5’-GGAGTCATCATCATCATCATCATC-3’ | 24bp |
| Materials | Volume |
| Fusion antibody receptor T vector (template) | |
| SIG_F | |
| IG_R | |
| dNTP (dNTP mixture 2.5mM TaKaRa) | |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | |
| 10x buffer (10x cloned Pfu Reaction buffer) | |
| DW (3rd sterile water) | |
| Total | 50ul |

Antibody (Submission)

| Primer | Direction | Sequences | Length |
| Ab_F | Forward | 5’-ACCCAGTCTCCAGCAATCATGTC-3’ | 23bp |
| Ab_R | Reverse | 5’-GGGGCTGTTGTTTTGGCTGAGG-3’ | 22bp |
| Materials | Volume |
| Fusion antibody receptor T vector (template) | |
| Ab_F | |
| Ab_R | |
| dNTP (dNTP mixture 2.5mM TaKaRa) | |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | |
| 10x buffer (10x cloned Pfu Reaction buffer) | |
| DW (3rd sterile water) | |
| Total | 50ul |

V_Fusion Antibody Receptor (Expression)
B_FGFR1 (Submission)
VB_FGFR1 (Expression)
Fusion Antibody Receptor (Expression)
5’UTR (expression)
3’UTR (expression)
Selection Marker (expression)
Core promoter + GFP (expression)
Proximal promoter (expression)
HA1 (expression)
HA2 (expression)
Fusion antibody receptor (expression)
5’UTR + Fusion Antibody receptor (expression)
HA1 + Selection Marker (expression)
HA2 + HR_R (expression)
5’UTR - Fusion Antibody receptor + HA1 - Selection Marker (expression)
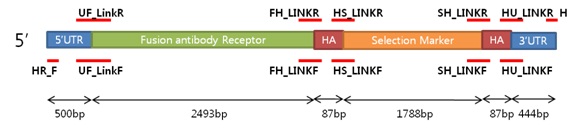
| Primer | Direction | Sequences | Length |
| HR_F(for homologous recombinant) | Forward | 5’-AAACCAGTTTTGCCAAAACAAC-3’ | 22bp |
| SH_LINKR | Reverse | 5’-GTCAGGAACATCGTATGGGTAGACCTGCAGAAGCTTAAGCTTG-3’ | 43bp |
| Materials | Volume |
| HA2 + HR_R (template), 5’UTR - Fusion Antibody receptor + HA1 - Selection Marker (template)
| |
| HR_F (for homologous recombinant) | |
| HR_R | |
| dNTP (dNTP mixture 2.5mM TaKaRa) | |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | |
| 10x buffer (10x cloned Pfu Reaction buffer) | |
| DW (3rd sterile water) | |
| Total | 50ul |

Homologous Recombinant (expression)

| Primer | Direction | Sequences | Length |
| HR_F(for homologous recombinant) | Forward | 5’-AAACCAGTTTTGCCAAAACAACTA-3’ | 24bp |
| HR_R | Reverse | 5’ – GCGTTCTTTTTTAATTCTGAATTAA – 3’ | 23bp |
| Materials | Volume |
| HA2 + HR_R (template), 5’UTR - Fusion Antibody receptor + HA1 - Selection Marker (template)
| |
| HR_F (for homologous recombinant) | |
| HR_R | |
| dNTP (dNTP mixture 2.5mM TaKaRa) | |
| Taq (Pfu Turbo® DNA Polymerase 2.5U/ μl STRATAGENE) | |
| 10x buffer (10x cloned Pfu Reaction buffer) | |
| DW (3rd sterile water) | |
| Total | 50ul |




September next
|
 "
"












