Team:Calgary/17 June 2010
From 2010.igem.org
| (4 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{CalgaryNotebookTemplate| | {{CalgaryNotebookTemplate| | ||
| - | + | Thursday June 17, 2010| | |
| - | + | ||
[[Image:06.17.2010_B0015+R0040_PCRGel.png|thumb|400px|Jeremy's gel electrophoresis of the colonies resulting from the construction of B0015 (double terminators) and R0040 (TetR repressible promoter)]] | [[Image:06.17.2010_B0015+R0040_PCRGel.png|thumb|400px|Jeremy's gel electrophoresis of the colonies resulting from the construction of B0015 (double terminators) and R0040 (TetR repressible promoter)]] | ||
| - | [[ | + | [[Image:06.18.2010.E1010-B0015.jpg|thumb|400px|Himika's gel electrophoresis of the colonies resulting from the construction of E1010 (Engineered Red Fluorescent Protein) and B0015 (double terminators)]] |
| + | |||
| + | <u>Emily</u> | ||
| + | |||
| + | Today I got sequencing results back for my I0500-B0034 construct. Unfortunately it does not look like the construction was succesful, as we couldn't find either part in the sequence. I restarted the construction, transformed it into TOP10 cells and plated on kanamycin for oveneright growth (with Dev's help) and tomorrow I shall start some more verification of these two parts seperately. Today I also looked into some more possible genes of interest for our various circuits. I also played around with creating a countdown to iGEM 2010 on our Wiki page. | ||
| + | |||
| + | |||
| + | <u>Dev, Chris, Jeremy</u> | ||
| + | |||
| + | Today, Jeremy restreaked the construct of B0015-R0040, did a PCR on it. He then ran a gel electrophoresis on the five colonies that grew. Colonies 1 and 5 are presumed to have the part desired as observed from the gel picture shown on the right. He also wrote a thank you letter to BioAlberta from which we received $1000 today. Dev helped Emily in her construction of I0500-B0034 through the Quick Ligase procedure. We are using presumed I0500 because we are unsure of the quality of the growth. I0500 has so far, failed to grow consistently. Chris redid the construction of J13002-E0032 to test the YFP from the Registry as well as attempting a plasmid switch of J23032 to a kanamycin resistant plasmid. | ||
| + | |||
| + | |||
| + | <u>Himika</u> | ||
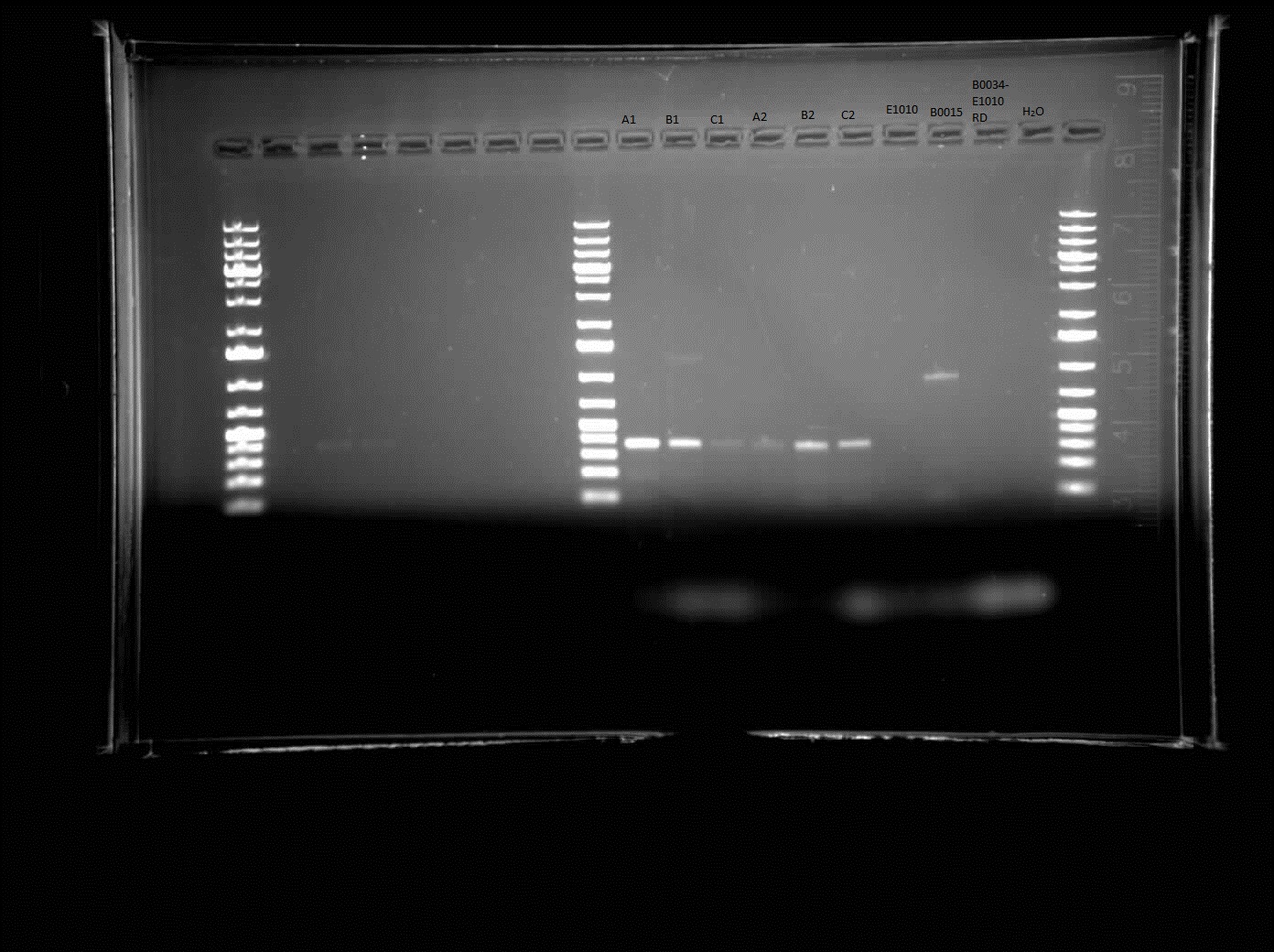
| - | + | Today, I did a colony PCR of the construct of E1010-B0015 and a gel electrophoresis of the colonies on the plate. The gel results came up as shown to the right. The bands that appeared were approximately 400 bp in size. The expected size was approximately double that of the size that appears. This indicates that the construction was unsuccessful or the PCR was unsuccessful. It should be redone. I also made overnight cultures of the colonies of E1010-B0015 before knowing that it was unsuccessful. These will be Miniprepped tomorrow and run on through agarose gel electrophoresis to check once again if it was successful or not. | |
| - | + | <u>Alex, Patrick, Raida</u> | |
| - | + | We did a new gel electrophoresis of the ''cpxP'' promoters that we isolated from the PCR product yesterday. The bottom half turned out quite dark, so we did an ethidium bromide stain of the gel, which made the image much clearer. The pieces are approximately 200bp long, where we expected approximately 161bp. Thus, we did a PCR purification of the product. We obtained two tubes with concentrations of 13.2 and 14.6 ng/μL. | |
}} | }} | ||
Latest revision as of 03:10, 23 August 2010

Thursday June 17, 2010
Emily
Today I got sequencing results back for my I0500-B0034 construct. Unfortunately it does not look like the construction was succesful, as we couldn't find either part in the sequence. I restarted the construction, transformed it into TOP10 cells and plated on kanamycin for oveneright growth (with Dev's help) and tomorrow I shall start some more verification of these two parts seperately. Today I also looked into some more possible genes of interest for our various circuits. I also played around with creating a countdown to iGEM 2010 on our Wiki page.
Dev, Chris, Jeremy
Today, Jeremy restreaked the construct of B0015-R0040, did a PCR on it. He then ran a gel electrophoresis on the five colonies that grew. Colonies 1 and 5 are presumed to have the part desired as observed from the gel picture shown on the right. He also wrote a thank you letter to BioAlberta from which we received $1000 today. Dev helped Emily in her construction of I0500-B0034 through the Quick Ligase procedure. We are using presumed I0500 because we are unsure of the quality of the growth. I0500 has so far, failed to grow consistently. Chris redid the construction of J13002-E0032 to test the YFP from the Registry as well as attempting a plasmid switch of J23032 to a kanamycin resistant plasmid.
Himika
Today, I did a colony PCR of the construct of E1010-B0015 and a gel electrophoresis of the colonies on the plate. The gel results came up as shown to the right. The bands that appeared were approximately 400 bp in size. The expected size was approximately double that of the size that appears. This indicates that the construction was unsuccessful or the PCR was unsuccessful. It should be redone. I also made overnight cultures of the colonies of E1010-B0015 before knowing that it was unsuccessful. These will be Miniprepped tomorrow and run on through agarose gel electrophoresis to check once again if it was successful or not.
Alex, Patrick, Raida
We did a new gel electrophoresis of the cpxP promoters that we isolated from the PCR product yesterday. The bottom half turned out quite dark, so we did an ethidium bromide stain of the gel, which made the image much clearer. The pieces are approximately 200bp long, where we expected approximately 161bp. Thus, we did a PCR purification of the product. We obtained two tubes with concentrations of 13.2 and 14.6 ng/μL.
 "
"