Team:Calgary/29 July 2010
From 2010.igem.org
| Line 2: | Line 2: | ||
'''Thursday July 29, 2010''' | '''Thursday July 29, 2010''' | ||
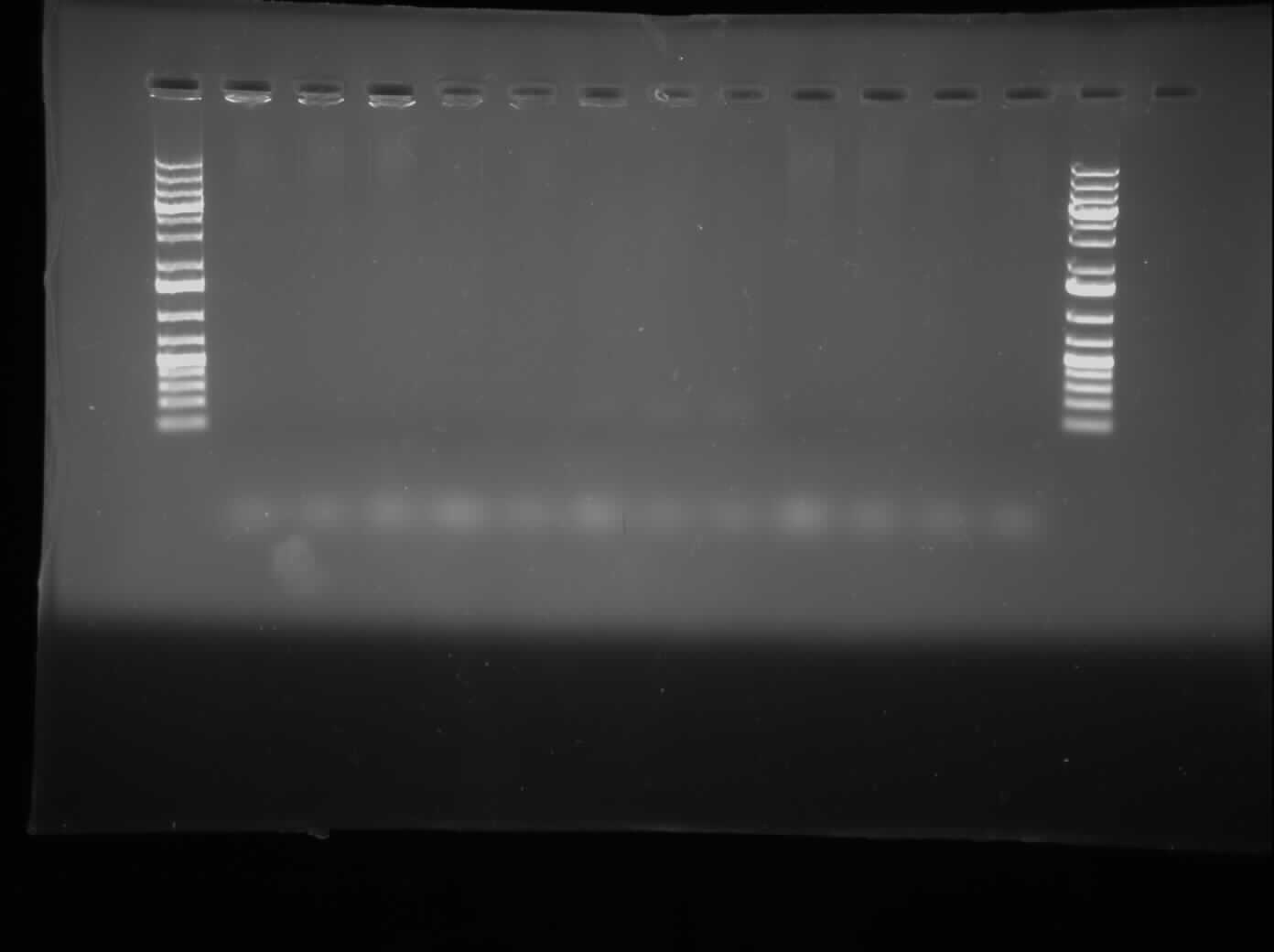
| - | + | [[Image:07.29.2010.ChrisCpxPFAILURE.jpg|thumb|400px|Chris's 1.0% gel electrophoresis of the PCR attempting to get CpxP promoter out of the <i>E. coli</i> genome]] | |
<u> Raida </u> | <u> Raida </u> | ||
Revision as of 19:57, 30 July 2010

Thursday July 29, 2010
Raida
Today I ran a 1% agarose gel electrophoresis of the PCR products that were done to amplify MalE and MalE31 genes with delete sequences and BBK-MalE and BBK-MalE31 primers. The results were negative as we saw no bands in lanes 1,4 and 5 as expected. Lanes 2 and 3 showed bands but there were other bands as well whic hindicated contamination. The bands were faint as well, so it was decided that the gel will be diregarded. Later during the day, Emily and I worked on a contstruction of MalE Gene (insert) which was isolated from the plasmid last week using gene specific primers with TOPO (vector). The purpose of doing this construction is to insert our gene of interest into a vector which is easy to work with and which has been used previously in the Calgary iGEM team and the compatibility was high. We also did the transformation and left it in the incubator and hope to see colonies tomorrow. Since the TOPO is Kan resistant, and the colonies will be growing in a Kanamiasine Plate, there will be no selection pressure. So they will be sent for sequencing to ensure that the insert and vector have ligated properly and that the constructions are successful.
Chris
Today, I ran a 1% gel of the PCR that was run to try to extract the CpxP promoter from the E. coli Top10 competent cell genome. The gel was run with all twelve tubes separate and it resulted in the gel picture shown on the right. While this was running, I did a PCR purification of the PCR product using a Qiagen kit. The gel showed that the PCR was unsuccessful and the tubes were discarded. A gradient PCR was set up with both differing temperatures (50°C-56°C) and differing MgCl2 concentrations of 1.5 µM, 2.0 µM, and 2.5 µM. The results of this will be run on a gel tomorrow. As well, I sent out a sponsorship letter to one of the University's premier sponsors: PepsiCo Imn.
No notebook page exists for this date. Sorry! "
"