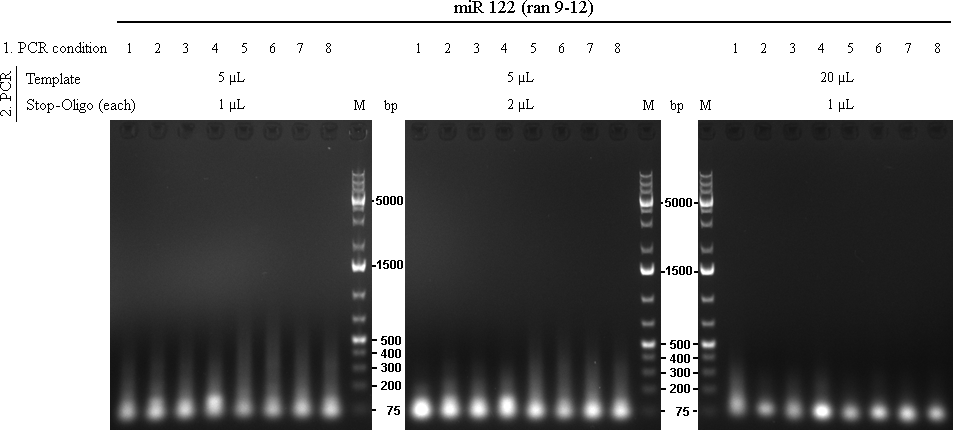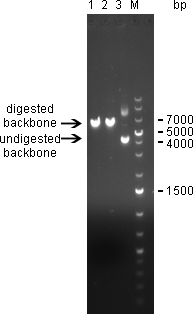Team:Heidelberg/Notebook/BSDesign/September
From 2010.igem.org
(→21/09/2010) |
(→25/09/2010) |
||
| (One intermediate revision not shown) | |||
| Line 536: | Line 536: | ||
When all constructs are in there, they could be cloned into their destination (miMeasure) vector using standard-cloning strategy. | When all constructs are in there, they could be cloned into their destination (miMeasure) vector using standard-cloning strategy. | ||
| + | |||
| + | Undigsted raPCR outcome of miR122 was amplified using [https://2010.igem.org/Team:Heidelberg/Notebook/Material/Primer#Primer_Table_raPCR ra017] and [https://2010.igem.org/Team:Heidelberg/Notebook/Material/Primer#Primer_Table_raPCR ra018]. | ||
| + | |||
| + | The PCR was then gel extracted using Qiagen Gel extraction protocol according to the 0S/0L/10S/10L scheme. | ||
| + | <br><br> | ||
---- | ---- | ||
== 24/09/2010 == | == 24/09/2010 == | ||
| + | |||
| + | Digestion of the PCR from 23/09/2010 with EcoRI and PstI in NEB EcoRI Buffer + BSA @37°C for 4h. The digestion was then purified using Qiagen PCR purification protocol. | ||
| + | |||
| + | Sample concentrations were determined. Molar ratio: backbone - inser -> 1:5 | ||
| + | |||
| + | Ligation was set up over night at 25°C using Fermentas T4 ligase according to procol | ||
<br><br> | <br><br> | ||
| + | ---- | ||
| + | |||
| + | == 25/09/2010 == | ||
| + | |||
| + | Transformation of miR-122 ligation to TOP10 E.coli. Cells were plated on LB-chloramphenicol-plates. | ||
| + | <br><br> | ||
| + | |||
| + | ---- | ||
| + | |||
| + | == 26/09/2010 == | ||
| + | |||
| + | Colony PCR using [https://2010.igem.org/Team:Heidelberg/Notebook/Material/Primer BBB-standard primer] was performed to screen for positive clones. | ||
| + | |||
| + | Over night culture of transformation from 25/09/2010 was set up (5mL LB-chloramphenicol-medium) for clones which could be positive: | ||
| + | 1: 0S<br> | ||
| + | 2: 0L<br> | ||
| + | 3: 10S<br> | ||
| + | 4: 10L<br> | ||
| + | |||
| + | *1.3, 1.5, 1.7, 3.8, 3.2 | ||
| + | |||
| + | ---- | ||
| + | |||
| + | == 27/09/2010 == | ||
| + | |||
| + | Plasmid preparation from over night culture using Qiagen Plasmid Minipreparation Kit. Samples were eluted in a total volume of 50µL water. | ||
---- | ---- | ||
Latest revision as of 03:44, 28 October 2010

Binding Site Design - September08-09/09/2010Oligo design for random assembly PCR (raPCR) Using endogenous miRNAs for cell-identification, a detectable difference in miRNA-expression levels need to be present. Therefore we sent isolated RNA from HeLa, HUH7 and HEK-293 cells either infected with AAV or non-infected to [http://www.febit.com febit] for microarray analysation of micro-RNA expression levels. The obtained results were delivered in an evaluated form, showing relative expression levels between the different cell lines and/or conditions. For further experiments, the highest relative differences of two approaches were looked up:
1. comparison in one cell line between control and AAV infected status
Positive or negative log-values indicate upregulation or downregulation in AAV infected cells compared to non-infected cells of the same cell type, respectively.
2. comparison in one condition between different cell linesHere are the most up- and downregulated miRNAs shown. One of those were picked for further experiments.
Beside this, it is known that hsa-mir-122 is expressed in liver uniquely, at least for humans. Additionally, mm-mir-375/376a are uniquely expressed in mouse livers. As we are heading for divergent prospective experiments, first we want to show that the principle of using endogenous miRNAs for tissue identification tasks is compatible with our constructs for luciferase measurements (compare to Synthetic miRNA-Kit) and our new measurement standard miMeasure. As it is known !!!reference!!! that multiple miRNA binding sites in a row increase the knock-down efficiency of miRNAs, binding patterns may help to find the right threshold for specific tissue targeting. Therefore we created a spacer sequence to seperate the single binding sites by rational design with a GC content of 50%. This spacer need to be as innert as possible, in terms of miRNA recognition, so the sequence was then tested for compatibility with other miRNAs. We found two 15bp-spacer, which make up a total 30bp-spacer, with low compatiblity to any miRNA given by the tools mentioned. Highest achieved mean free energy was higher than -30 kcal/mol (the higher, the less effective is the binding), whereas a perfect binding miRNA was, in our cases, always under -40 kcal/mol. For our random assembly PCR approach, we created oligos using this spacer-sequence as annealing site. Therefore we split the spacer in two halfs, and placed a miRNA binding site in between:
Spacer sequence:
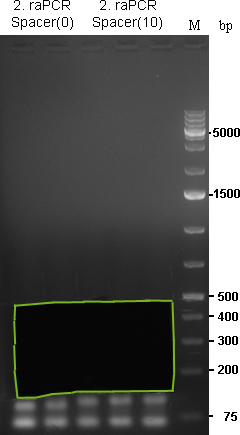
Additionally we created Spacer sequences with 10 or 20 nucleotides between the two halves. If we have time, we could then analyse the effect of the spacer sequence, additionially on the binding site pattern. Oligos were ordered corresponding to the Primer Table
13/09/2010
Assay:
Restriction digest was performed for approx. 5h
stop-oligos: raPCR_AS13-stop_rev_NotI and raPCR_AS13-stop_fw_XhoI (ra017/018) Oligos were used in standard conc. (100µM)
for the next PCR, three assay will tried:
stop-oligos: raPCR_AS13-stop_rev_NotI and raPCR_AS13-stop_fw_XhoI
In total there were 72 reactions. Each was run with 2x Phusion Mastermix, missing volume was filled with water.
DNA was stored in fridge afterwards
14/09/2010The 72 PCRs from 13/10/2010 were analysed on 1% agarose gel.
As we are looking for multiple binding sites, lanes with longest smear, meaning more long binding sites, were choosen for preparative gel:
As the gel volume was too much for dissolving in a single 2 mL tube, each part was splitted for dna extraction and brought together on the column. Gel extractions were done according to Qiagen Gel-extraction kit protocal and eluted in 30 µL water.
15/09/2010Sample code:
First row of cloning will be done with miR-122 samples. Others will follow. Samples prepared: 122-0S,-0L,-10S,-10L Gel extracted samples were digested with NotI/XhoI for cloning into psiCheck-2 vector: 5µL DNA (2µL for backbone) in a total volume of 30µL using 1µL XhoI and 0.6µL NotI enzyme, for 1.5 h at 37°C. The digested DNA was then purified using Qiagen nucleotide removal kit and eluted in 30µL. Subsequently, digested fragments were ligated over night at room temperature. Ligation assay for Fermentas T4 ligase: 2µL Buffer 1µL Ligase 7µL water 1µL Backbone (6000bp, psiCHECK-2) 9µL purified restriction digest
16/09/2010Transformation of ligations: 5µL ligation assay in 50µL TOP10 E.coli 25 min on ice 45sec heat shock on 42°C 1.5-2h shaking at 37°C plated 200µL on Ampicillin-LB/Agar-Plates after incubating ~8h, at 37°C, the plates were incubated overnight at room temperature
17/09/2010Colonies were visible in reasonable numbers on every plate Colony PCRs were performed to check for positive clones. Primer for colony PCR were stop-oligos, used in the raPCR. The PCR was performed in a total volume of 20 µL. One colony was dissolved in 20µL water. 5µL of this bacteria solution was used as PCR template. PCR conditions as recommended from Fermentas (see link above). From each plate, 10 colonies were picked (40 in total). Colony PCRs were then analysed on 1.5% agarose gel. Result: all negative Troubleshooting.... Minipreps were prepared (5mL - LB-ampicillin) for each sample for text digestion (over night, shaking @37°C)
18/09/2010Plasmid DNA was purified from over night cultures using Qiagen Plasmid Miniprep Kit according to the protocol. Elution was performed in 30 µL water. Concentrations ranged from approx. 400 to 788 ng/µL. Test digestion with NotI/XhoI was performed for 1h at @37°C and analysed on an 1.5% agarose gel. No insert was visible.
19/09/2010Troubleshooting: Possible Problems
Testing steps:
Over night digestion of backbone was performed at 37°C. 0.5 µg DNA was digested with 1 µL Enzyme in NEB Buffer 3 + BSA in a total volume of 30µL
20/09/2010Digestion of psiCHECK-2 plasmid was analysed on 1% agarose gel: Here we can see that both enzymes cut the plasmid. The linearized vector (visible at 6 kpb) in general shows up as a higher band than its undigested version, which is here visible at 4 kbp. Where the undigested plasmid shows concatemers, those are not visible after digestion, which proofs again for succesful digestion. The new digested plasmid-backbone was used for repeat of the ligation.
21/09/2010
Ligation was performed for 4h @25°C (1µL NotI/XhoI-linearised psiCHECK2-plasmid + 4µL digested raPCR product) using Fermentas T4 Ligase. Afterwards they were transformed into TOP10 E. coli and grown over night in LB-Ampicillin-medium
22/09/2010Plasmid DNA was extracted from over night cultures using Qiagen Plasmid Miniprep Kit. DNA was then analysed via test-digestion with NotI/XhoI Enzymes. Test digestion was performed in 20 µL using 0.4µL Enzyme, supplied with NEB buffer 3 and BSA for 1h @ 37°C. Test digestion was then analysed on a 2% agarose gel. No sample showed any insert....
23/09/2010strategy change -> psiCHECK2-plasmid is somehow not working from now on, all samples will be cloned into the standard plasmid PSB1C3-P1010. When all constructs are in there, they could be cloned into their destination (miMeasure) vector using standard-cloning strategy. Undigsted raPCR outcome of miR122 was amplified using ra017 and ra018. The PCR was then gel extracted using Qiagen Gel extraction protocol according to the 0S/0L/10S/10L scheme.
24/09/2010Digestion of the PCR from 23/09/2010 with EcoRI and PstI in NEB EcoRI Buffer + BSA @37°C for 4h. The digestion was then purified using Qiagen PCR purification protocol. Sample concentrations were determined. Molar ratio: backbone - inser -> 1:5 Ligation was set up over night at 25°C using Fermentas T4 ligase according to procol
25/09/2010Transformation of miR-122 ligation to TOP10 E.coli. Cells were plated on LB-chloramphenicol-plates.
26/09/2010Colony PCR using BBB-standard primer was performed to screen for positive clones. Over night culture of transformation from 25/09/2010 was set up (5mL LB-chloramphenicol-medium) for clones which could be positive:
1: 0S
27/09/2010Plasmid preparation from over night culture using Qiagen Plasmid Minipreparation Kit. Samples were eluted in a total volume of 50µL water. 28/09/2010miR-122 samples were sent for sequencing (by GATC)
29/09/2010
30/09/2010
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"