Team:Imperial College London/Schistosoma
From 2010.igem.org
(→Treatment) |
|||
| (23 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{:Team:Imperial_College_London/Templates/Header}} | {{:Team:Imperial_College_London/Templates/Header}} | ||
| + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:25px;" cellspacing="20" | ||
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Schistosoma | ||
| + | |- | ||
| + | |'''Our system turns yellow when a ''Schistosoma'' parasite, which causes schistosomiasis or bilharzia, is present. It is the most devastating water-borne parasite in the world and causes huge problems in many developing countries. For more information, read on...''' | ||
| + | |} | ||
| + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | ||
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Schistosoma | ||
| + | |- | ||
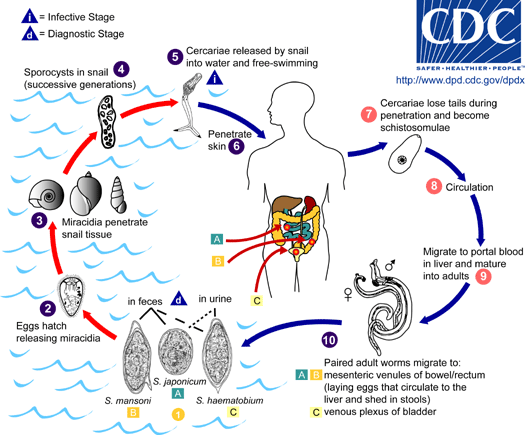
| + | |[[Image:Schistomes LifeCycle.gif|thumb|right|350px| Life cycle of the schistosome parasite [http://www.dpd.cdc.gov/dpdx/HTML/Schistosomiasis.htm CDC (2010)]]] | ||
| - | + | ''Schistosoma'', from the class of trematodes constitute a genus commonly known as blood-flukes, are the parasites causing a disease called bilharzias or schistosomiasis which, according to the [http://www.who.int/mediacentre/factsheets/fs115/en/index.html WHO (2010)] is amongst the most devastating parasitic disease, second only to malaria, with 207 million infected and around 700 million at risk. | |
| - | + | The most significant species of ''Schistosoma'' are ''S. haematobium'', ''S. mansoni'', and ''S. japonicum'', which combined cause a loss of over 1.5 million life years. Schistosomiasis shows typically a focal epidemiology and uniformly distributed amongst populations, with higher infection rates in children than in adults. Acute schistosomiasis, including symptoms such as fever and malaise, is most commonly found in primary infected individuals whereas chronic schistosomiasis and its accompanying complications such as hepatic or intestinal schistosomiasis, are more predominant in people with long lasting infection mainly in poor rural areas. This is caused by a slow process of immune acquisition as well as the long lasting effect of schistosomal eggs trapped in tissues leading to inflammatory reactions. Diagnosis is usually accomplished by microscopy of stool samples although in many cases, especially in rural settings, treatment with Praziquantel is done without individual diagnosis. Much progress has been made in the control of the disease through community-based chemotherapy however these rely on political commitment and strong health systems. To understand the disease itself, as well as the possible approaches to improving its control we have to gain a deeper understanding of the complex life cycle of this parasite. Like many other eukaryotic parasites, the ''Schistosoma'' life cycle involving several stages in and outside two different hosts. | |
| - | |||
| - | + | '''The Life Cycle''' | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
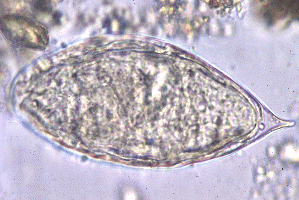
Infected people can pass out several thousands of parasite eggs in their stool (most species) or urine (''Schistosoma haematobium''). These eggs were first discovered in Egypt by Theodor Maximilian Bilharz, a German pathologist. Under the microscope one can see that they are around 110-170 µm long by 40 to 70 µm wide giving them an elongated shape with a distinctive terminal spine that is used for identification. | Infected people can pass out several thousands of parasite eggs in their stool (most species) or urine (''Schistosoma haematobium''). These eggs were first discovered in Egypt by Theodor Maximilian Bilharz, a German pathologist. Under the microscope one can see that they are around 110-170 µm long by 40 to 70 µm wide giving them an elongated shape with a distinctive terminal spine that is used for identification. | ||
| - | [[Image:Schistosoma egg.png|thumb|right|200px| Egg of | + | [[Image:Schistosoma egg.png|thumb|right|200px| Egg of ''S. haematobium''. [http://www.stanford.edu/class/humbio103/ParaSites2004/Schisto/website.html Stanford University (2004)]]] |
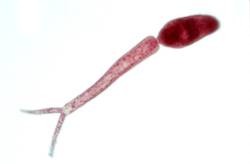
[[Image:Cercaria.jpg|thumb|right|200px| Schistosomal cercaria. [http://biology.unm.edu/biology/esloker/pi/SchistoEvolHistory.htm Loker Laboratory (2006)]]] | [[Image:Cercaria.jpg|thumb|right|200px| Schistosomal cercaria. [http://biology.unm.edu/biology/esloker/pi/SchistoEvolHistory.htm Loker Laboratory (2006)]]] | ||
| - | These characteristically shaped eggs are viable for around week and once in contact with water they quickly hatch and release the first larval form called miracidia. These free-swimming, ciliated larvae cannot feed and will usually die within 24 hours if they fail to detect a suitable intermediary host, using light and chemical | + | These characteristically shaped eggs are viable for around week and once in contact with water they quickly hatch and release the first larval form called miracidia. These free-swimming, ciliated larvae cannot feed and will usually die within 24 hours if they fail to detect a suitable intermediary host, using light and chemical stimuli, and subsequently enter it. Different ''Schistosoma'' species show preference for different host species but at this stage all rely on fresh water snails. |
Once inside the host the miracidia reproduce asexually and form multicellular sporocysts which later develop into cercarial larvae with embryonic suckers and a characteristic bifurcated tail. | Once inside the host the miracidia reproduce asexually and form multicellular sporocysts which later develop into cercarial larvae with embryonic suckers and a characteristic bifurcated tail. | ||
| - | This process takes between 4 and 6 weeks from which time on thousands of cercaria, a second larval form, can be shed from the snails daily for several month. | + | This process takes between 4 and 6 weeks from which time on thousands of cercaria, a second larval form, can be shed from the snails daily for several month. This shedding is induced by light [http://www.ncbi.nlm.nih.gov/pubmed/16997665 (Gryseels ''et al.'' 2006)] and aims to maximise the likelihood of cercaria finding their second host. The larvae can survive in the water for up to 72 hours, although more commonly around 30 hours, during which time they use chemical stimuli as well as light and temperature to detect the skin of a suitable host. By far the most powerful stimulus to triggering invasive behaviour are skin lipids, in particular medium-chain free fatty acids [http://www.ncbi.nlm.nih.gov/pubmed/11983589 (McKerrow and Salter 2002)]: Of the C18 fatty acids examined, stearic (18:0) is inactive, oleic (18:1) slightly active, linoleic (18:2) and linolenic (18:3) acids highly active [http://journals.cambridge.org/action/displayAbstract;jsessionid=B1A531CD8298B78F36E5A5A336522AD3.tomcat1?fromPage=online&aid=4180708 (Austin ''et al.'' 1974)]. Once detected by the parasite these stimuli lead to signal-dependent breakdown of inositol phospholipids which is directly linked to activation of protein kinase C (via elevated diacylglycerol level) and mobilization of calcium (via elevated levels of inositol triphosphate) which in turn evokes subsequent cellular response [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WFH-4C52GG2-FP&_user=217827&_coverDate=04%2F30%2F1991&_alid=1505009318&_rdoc=1&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=6795&_sort=r&_st=13&_docanchor=&view=c&_ct=4&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=b5b1e3b6d044a907895dc267d542cf09&searchtype=a (Matsumura ''et al.'' 1991)] such as the release of enzymes from a specialized gland - called acetabular gland complex – at the posterior region of the head of the parasite [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WFH-4C52GPD-KH&_user=217827&_coverDate=08%2F31%2F1992&_alid=1505013773&_rdoc=2&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=6795&_sort=r&_st=13&_docanchor=&view=c&_ct=8&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=273e73fe67245cd2feb84a18cc1fbbec&searchtype=a (Fishelson ''et al.'' 1992)]. |
| - | These enzymes include number of proteases that target proteins in the host's skin such as keratin and elastin the latter of which is cleaved by the most essential and abundant protease called schistosomal elastase, which we use in our iGEM project to detect | + | These enzymes include number of proteases that target proteins in the host's skin such as keratin and elastin the latter of which is cleaved by the most essential and abundant protease called schistosomal elastase, which we use in our iGEM project to detect ''Schistosoma''. This process allows cercaria to burrow through the skin of their new host, where they leave their tail behind, into blood vessels. |
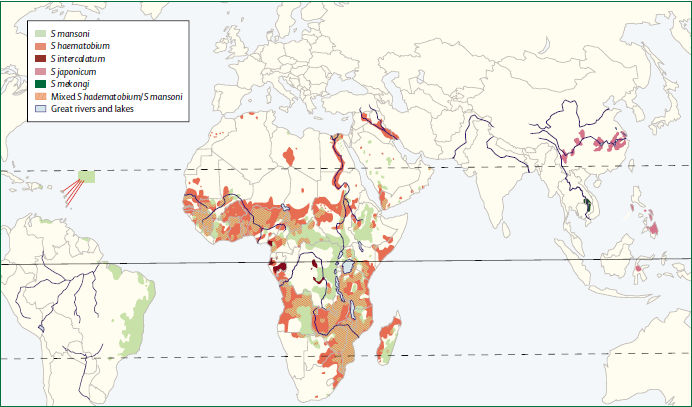
| - | There are six species of | + | There are six species of ''Schistosoma'' known to successfully infect humans: ''Schistosoma mansoni'' , which uses ''Biomphalaria'' snails as intermediate host and can cause intestinal and hepatic schistosomiasis in Africa, the Arabian peninsula as well as South America; ''S. japonicum'', a zoonotic parasite, which uses ''Oncomelania'' snails as intermediate host and can cause intestinal and hepatosplenic schistosomiasis, especially in South-East Asia; ''S. mekongi'', ''S. intercalatum'' and related ''S. guineansis'' ,which are restricted to small areas an only of local importance; as well as ''S. haematobium'', transmitted by ''Bulinus'' snails, which can give rise to urinary schistosomiasis in Africa and the Arabian peninsula |
| - | [[Image:Spread of schistosoma species (Gryseels et al. 2006).png|thumb|center|750px| Map of the distribution and spread of | + | [[Image:Spread of schistosoma species (Gryseels et al. 2006).png|thumb|center|750px| Map of the distribution and spread of ''Schistosoma'' species. [http://www.ncbi.nlm.nih.gov/pubmed/16997665 (Gryseels ''et al.'' 2006)] ]] |
| - | Additionally there is number of avian | + | Additionally there is number of avian ''Schistosoma'' species, especially in Northern Europe, the USA and Canada that cannot infect humans but will still try to enter our skin giving rise to the harmless but unpleasant condition called swimmer's itch. However these conditions, like cercarial dermatitis often go unnoticed in endemic areas. |
| - | Once inside the human body, the cercaria travel through the blood via the lungs into the portal vein of the liver where they develop into schistosomulae. After a maturation period one to one and a half month after which they mate. The way | + | Once inside the human body, the cercaria travel through the blood via the lungs into the portal vein of the liver where they develop into schistosomulae. After a maturation period one to one and a half month after which they mate. The way ''Schistosoma'' have evolved to mate is unique amongst the trematodes in that there is a significant sexual dimorphism, which also inspired their name which translates from Greek to 'split body'. This name describes the male anatomy as there is a long groove, called gynecophoral canal, along its body in which the female is held. |
| - | Adult males are between 10 to 15 mm long whereas females are longer, between 16 to 22mm, and thinner. Both sexes have two suckers – one on each end – to hold onto the walls of blood vessels where they feed on blood and globulins through anaerobic glycolysis. The debris is regurgitated in the host’s blood. After mating they migrate to their perivesicular (''S. haematobium'') or mesenteric (other species) destination where the female releases her eggs. With an average life span of 3 to 5 years, although up to 30 years is possible, | + | Adult males are between 10 to 15 mm long whereas females are longer, between 16 to 22mm, and thinner. Both sexes have two suckers – one on each end – to hold onto the walls of blood vessels where they feed on blood and globulins through anaerobic glycolysis. The debris is regurgitated in the host’s blood. After mating they migrate to their perivesicular (''S. haematobium'') or mesenteric (other species) destination where the female releases her eggs. With an average life span of 3 to 5 years, although up to 30 years is possible, ''The theoretical reproduction potential of one schistosome pair is up to 600 billion schistosomes'' [http://www.ncbi.nlm.nih.gov/pubmed/16997665 (Gryseels ''et al.'' 2006)]. |
| - | [[Image:Schistosoma2.png|thumb|right|350px| Pictures of | + | [[Image:Schistosoma2.png|thumb|right|350px| Pictures of ''Schistosoma''. Top: Electron microscopy of ''Schistosoma'' couple [http://observationsofanerd.blogspot.com/2009/06/this-weeks-sci-fi-worthy-parasite.html Link]. Bottom: Artistic impression of schistosome parasite in blood vessel [http://animal.discovery.com/invertebrates/monsters-inside-me/schistosomiasis-schistosoma-mansoni/ Link].]] |
The eggs themselves secrete proteolytic enzymes that allow the eggs to reach their destination: the bladder (''S. Haematobium'') or intestines (other species) from which they are released by the host into the environment, allowing the cycle to continue. | The eggs themselves secrete proteolytic enzymes that allow the eggs to reach their destination: the bladder (''S. Haematobium'') or intestines (other species) from which they are released by the host into the environment, allowing the cycle to continue. | ||
| - | |||
| - | + | '''Impact on human health - Acute symptoms''' | |
| - | The first signs of infection can often be observed directly after penetration of the skin causing a temporary rash that can | + | The first signs of infection can often be observed directly after penetration of the skin causing a temporary rash that can persist for days as paulopruriginous lesions. This occurs in particular after primary infection with the parasite frequently found in migrants into and tourists returning from endemic areas as a study by [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WJT-4H4T0M2-3&_user=217827&_coverDate=05%2F31%2F2006&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=e7f6675f5954192c9e6b1c1b64d6a484&searchtype=a Bottieau et al. 2006 ] showed. The more progressive stages of schistosomiasis, often referred to as Katayama fever, are also rarely diagnosed in the endemic population, maybe as a result of under diagnosis, but more commonly found in migrants and tourists also. The trained immune system of healthy people living in endemic areas will often be able to fight off parasite infections naturally. Furthermore cord blood lymphocytes from newborns of schistosome-infected mothers are significantly more likely to amount a helminth Ag-driven B cell response than others, indicating ''in utero'' sensitisation takes place [http://www.jimmunol.org/cgi/content/full/160/7/3578 (King et al. 1998)]. Acute schistosomiasis is a systemic hypersensitivity reaction caused by the migration of schistosomulae within the host body and usually weeks or even months after primary infection. It is characterised by a sudden onset of symptoms ranging from fever, fatigue, and myalgia over malaise, non-productive cough, eosinophilia and patchy infiltrates on chest radiography. These can later also be accompanied by abdominal symptoms caused by movement of mature worms. |
| - | + | ||
| + | '''Impact on human health - Long-term effects''' | ||
Chronic pathology caused by schistosome infection is rarely evoked by the adult worms, but rather by lesion caused by the eggs that become trapped in the tissue during perivesical or perintestinal migration, or in other cases after embolisation by various organs including the liver, spleen, lung and cerebrospinal system. As a result of protease secretion, an eosinophilic inflammatory response and granulomatous reaction are provoked which are later replaced by fibrous deposits. The granulomatous responses are "strictly mediated by CD4+ T helper (Th) cells specific for egg | Chronic pathology caused by schistosome infection is rarely evoked by the adult worms, but rather by lesion caused by the eggs that become trapped in the tissue during perivesical or perintestinal migration, or in other cases after embolisation by various organs including the liver, spleen, lung and cerebrospinal system. As a result of protease secretion, an eosinophilic inflammatory response and granulomatous reaction are provoked which are later replaced by fibrous deposits. The granulomatous responses are "strictly mediated by CD4+ T helper (Th) cells specific for egg | ||
antigens" [http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0036-46652005000400001&lng=en&nrm=iso&tlng=en Nascimento-Carvalho & Moreno-Carvalho 2005]. The extent to which an individual suffers is therefore directly dependent on the disease burden but also the precise immune response mounted by the individual. Depending on the species of schistosome a person is infected with, a number of distinct conditions can be found, if the disease enters the chronic stages and is not treated timely. | antigens" [http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0036-46652005000400001&lng=en&nrm=iso&tlng=en Nascimento-Carvalho & Moreno-Carvalho 2005]. The extent to which an individual suffers is therefore directly dependent on the disease burden but also the precise immune response mounted by the individual. Depending on the species of schistosome a person is infected with, a number of distinct conditions can be found, if the disease enters the chronic stages and is not treated timely. | ||
| - | In addition to the immunopathology that can be attributed directly to the parasite, it is although thought to be a major factor contributing to | + | In addition to the immunopathology that can be attributed directly to the parasite, it is although thought to be a major factor contributing to morbidity and accompanying problems such as anaemia, nutritional status and cognitive and physiological capacity, as well as fatigue [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T1B-4G2BRY1-12&_user=217827&_coverDate=05%2F06%2F2005&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_searchStrId=1510251595&_rerunOrigin=google&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=505520bc94a7d520db8ae3abeb1033b1&searchtype=a (King ''et al.'' 2005)]. Older studies failed to demonstrate this effect even in individuals, but more recent studies have shown a small, yet significant, effect of the parasite on human health in general. |
'''Urinary schistosomiasis''' | '''Urinary schistosomiasis''' | ||
| - | This complication caused by | + | This complication caused by ''Schistosoma'' is most notably found in young children between 5 and 10 and is rare during and after adolescence. The eggs of ''S. haematobium'', which are excreted via urine as part of the parasite life cycle, can be trapped in the genitourinal system and elicit a granulomatous inflammation, ulceration or pseudopolyposis both in adjacent blood vessels and the urethral walls. The early symptoms include dysuria, pollakisuria, proteinuria and most importantly haematuria. The later can vary from small amount of blood found in the terminal urine to darker colouration of the whole urine sample in more severe cases and the condition can often be exacerbate by bacterial superinfections or bladder stones. The proportion of untreated infected individuals with bladder complication to those without seems to vary significantly (between 0% and 97%) however after treatment with Praziquantel a full recovery is made in most cases. Notably this suggests that the renal parenchyma is usually compressed but not destroyed although schistosomiasis-induced renal damage can be fatal particularly for older patients [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T1B-4KY3RPD-M&_user=217827&_coverDate=09%2F29%2F2006&_alid=1505007608&_rdoc=5&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=4886&_sort=r&_st=13&_docanchor=&view=c&_ct=9&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=27498d6154512f9cccda7ff0d2fbb805&searchtype=a (Gryseels ''et al.'' 2006)]. |
| - | Furthermore a study by [http://www.nature.com/search/executeSearch?sp-q-1=BJC&sp-q=Schistosomiasis+and+the+risk+of+bladder+cancer+in+Alexandria%2C+Egypt&sp-c=25&sp-m=0&sp-s=date_descending&include-collections=journals_nature%2Ccrawled_content&exclude-collections=journals_palgrave%2Clab_animal&sp-a=sp1001702d&sp-sfvl-field=subject|ujournal&sp-x-1=ujournal&sp-p-1=phrase&sp-p=all&submit=go Bedwani et al. 1998] also found urinary schistosomiasis to be a significant contributor to bladder cancer accounting for around 16% of the cases amongst the Egyptian | + | Furthermore a study by [http://www.nature.com/search/executeSearch?sp-q-1=BJC&sp-q=Schistosomiasis+and+the+risk+of+bladder+cancer+in+Alexandria%2C+Egypt&sp-c=25&sp-m=0&sp-s=date_descending&include-collections=journals_nature%2Ccrawled_content&exclude-collections=journals_palgrave%2Clab_animal&sp-a=sp1001702d&sp-sfvl-field=subject|ujournal&sp-x-1=ujournal&sp-p-1=phrase&sp-p=all&submit=go Bedwani et al. 1998] also found urinary schistosomiasis to be a significant contributor to bladder cancer accounting for around 16% of the cases amongst the Egyptian population and the [http://monographs.iarc.fr/ENG/Monographs/vol61/volume61.pdf IARC (1997)] found that there is sufficient evidence for the carcinogenicity of infection with ''S. haematobium''. |
'''Intestinal schistosomiasis''' | '''Intestinal schistosomiasis''' | ||
| - | Once again the eggs of the parasite induce an immune response in the form of mucosal granulomatous inflammation, pseudopolyposis, microulcerations, and superficial bleeding, with lesions mostly located in the large bowel and rectum. Symptoms include chronic as well as intermittent abdominal pain and discomfort, loss of appetite, diarrhoea, and bloody faeces. This condition manifests itself in chronically infected individuals at a relatively moderate level between 3-55% with 30-60% of diarrhoea cases attributable to | + | Once again the eggs of the parasite induce an immune response in the form of mucosal granulomatous inflammation, pseudopolyposis, microulcerations, and superficial bleeding, with lesions mostly located in the large bowel and rectum. Symptoms include chronic as well as intermittent abdominal pain and discomfort, loss of appetite, diarrhoea, and bloody faeces. This condition manifests itself in chronically infected individuals at a relatively moderate level between 3-55% with 30-60% of diarrhoea cases attributable to ''Schistosoma'' infection. However the statistical significance of these numbers is debatable as sampling techniques have varied across different studies. |
'''Hepatic schistosomiasis''' | '''Hepatic schistosomiasis''' | ||
| - | This is one of the most significant complications | + | This is one of the most significant complications ''Schistosoma'' can cause during chronic infection, which can cause long-lasting, potentially fatal health problems, even after treatment with Praziquantel. It is caused by ''S. mansoni'', ''S. japonicum'' and ''S. mekongi''. Technically early inflammatory and late fibriotic hepatic disease, also called Symmers' clay-pipestem fibrosis of the liver, are two distinct conditions, in terms of morbidity, clinical practise and immunological mechanism, although they are combined here as hepatic (and hepatosplenic) schistosomiasis [http://www.ajtmh.org/cgi/content/abstract/17/1/38 Cheever (1968)]. |
| - | A mild inflammation is most common in young individuals especially during the early stages of infection and can be observed in around 80% of infected children. The main cause of hepatomegaly are ova which got caught in the presinusoidal periportal spaces of the liver, giving rise to sharp-edged enlargements of the left liver lobe as well as nodular splenomegaly reaching from the costal arch to the umbilicus or in extreme cases even to the pelvis. During chronic infection with | + | A mild inflammation is most common in young individuals especially during the early stages of infection and can be observed in around 80% of infected children. The main cause of hepatomegaly are ova which got caught in the presinusoidal periportal spaces of the liver, giving rise to sharp-edged enlargements of the left liver lobe as well as nodular splenomegaly reaching from the costal arch to the umbilicus or in extreme cases even to the pelvis. During chronic infection with ''Schistosoma'' however fibrotic or chronic hepatic schistosomiasis can arise in a small proportion of mostly young to middle aged adults in particular those with a high parasite load, although immunogenetic predispositions are thought to be the most significant risk factor [http://www.jimmunol.org/cgi/search?sortspec=relevance&author1=Chevillard&fulltext=schistosomiasis&pubdate_year=2003&volume=&firstpage= (Chevillard ''et al.'' 2003)]. The disease is caused by heavy deposition of diffuse collagen in the periportal space leading to a hardening of the liver and obstruction of blood flow through the portal veins, which can result in portal hypertension, splenomegaly, and others including commonly fatal bleedings in that latest stages [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B75J9-4G7WVDX-7&_user=217827&_coverDate=09%2F01%2F2000&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_searchStrId=1509470188&_rerunOrigin=scholar.google&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=a872e3647a4e62a95325c92be7470219&searchtype=a (Bica ''et al. 2000)]. |
| - | The liver complications caused by | + | The liver complications caused by ''S. japonicum'' can progress much faster from early mild to chronic stages resulting in sudden onset of bleeding. |
'''Neuroschistosomiasis''' | '''Neuroschistosomiasis''' | ||
| - | This condition is the second most common presentation of infection by | + | This condition is the second most common presentation of infection by ''S. mansoni'' also causing granulomatous inflammatory reaction due to schistosome eggs reaching the spinal cord or brain via the vascular system, mainly through "arterial or retrograde venous blood flow via the valveless perivertebral plexus of Batson", or by occasional migration of adult schistosomes. The main clinical syndromes are spinal cord neuroschistosomiasis divided into acute or subacute myelopathy and localized cerebral or cerebella neuroschistosomiasis. The later gives rise to focal CNS impairment, seizures as well as increased intracranial pressure. Presumptive diagnosis of neuroschistosomiasis relies on confirmation of the presence of ''S. mansoni'' infection by stool microscopy for trematode eggs, and also serologic testing of blood and spinal fluid. |
Because this condition is relatively rare in Western countries, it is often hard to diagnose. It should be considered if a patient | Because this condition is relatively rare in Western countries, it is often hard to diagnose. It should be considered if a patient | ||
| Line 80: | Line 84: | ||
'''Ectopic schistosomiasis''' | '''Ectopic schistosomiasis''' | ||
| - | Schistosomiasis can | + | Schistosomiasis can less frequently also cause complications in other organs, such as lung or the genitalia. These as well are caused by the parasite eggs and the ensuing inflammatory reaction. Lesions in the genital areas such as vulva and vagina might have implications for sexual transmission, although this is not well documented. Additionally there is the risk of infertility in those cases where lesions and inflammations occur near the testicles or the fallopian tube and ovaries. |
| - | |||
| - | '''Praziquantel''' | + | '''Treatment - Praziquantel''' |
| - | There are a number of drugs available to cure infection with schistosomes, for example amoscanate, chloroxylenol, meclonazepam and oxamniquine. However the by far most widely used helminticide to combat schistosomiasis is Praziquantel despite not being licensed for medical treatment of humans in the UK. It is effective | + | There are a number of drugs available to cure infection with schistosomes, for example amoscanate, chloroxylenol, meclonazepam and oxamniquine. However the by far most widely used helminticide to combat schistosomiasis is Praziquantel despite not being licensed for medical treatment of humans in the UK. It is effective against ''Schistosoma'' in a single treatment, although it should be followed up by a second treatment after 4-6 weeks, and is also used for some other parasitic trematodes such as liver flukes. Although the drug has been in use for over 20 years, the mechanism of its action is still not understood [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T29-4V1KMJN-1&_user=217827&_coverDate=03%2F31%2F2009&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=d2d7b0f7be37d4568b69a5b61438f7d8&searchtype=a (Aragon ''et al.'' 2009)]. Side effects are usually mild, although in cases with high parasite load they can be more severe as a result of massive release of parasitic antigens from the dead worms. |
''"Praziquantel is the recommended treatment for schistosomiasis at 40 mg/kg body weight. The cost of a single 600-mg tablets is about US$ 0.08 and an average treatment is estimated to be between US$ 0.20–0.30. Praziquantel is now available free of charge to a few high-disease burden least developed countries (LDC), through a donation from Merck KGaA to the World Health Organization."'' [http://www.who.int/schistosomiasis/strategy/en/index.html (WHO, 2010)] | ''"Praziquantel is the recommended treatment for schistosomiasis at 40 mg/kg body weight. The cost of a single 600-mg tablets is about US$ 0.08 and an average treatment is estimated to be between US$ 0.20–0.30. Praziquantel is now available free of charge to a few high-disease burden least developed countries (LDC), through a donation from Merck KGaA to the World Health Organization."'' [http://www.who.int/schistosomiasis/strategy/en/index.html (WHO, 2010)] | ||
| - | '''Communal approach to treatment''' | + | '''Treatment - Communal approach to treatment''' |
| + | |||
| + | Because of the difficult nature of schistosomiasis diagnosis, treatment is often not provided after individual diagnosis, but instead it is very common to test only a sample of individuals in a community, especially school children, and if a certain proportion of the sampled people is infected, the whole community is treated with Praziquantel. This measure allows control of the disease to some extend if done in a coordinated approach with treatment on a large scale with safe and effective drug, and at regular intervals [http://www.who.int/schistosomiasis/strategy/en/index.html (WHO, 2010)]. The communal approach aims at reducing the late stages of schistosomiasis that go along with malnutrition and often cause developmental problem in children. Based on the sampled disease burden is also the length of the period after which treatment is repeated. This method will not eradicate the disease but rather to lower the total number of infected people and prevent severe infection in order to reduce morbidity as well as socio-economic impact. In theory it is also possible to break the parasitic life cycle with this method and permanently prevent reinfection, however in practise it is well known that eradication does not take place for a number of different reasons, which is why treatment is repeated regularly. Even if ''Schistosoma'' were completely eradicated from one area, due to the continuing presence of the water snail vector, recolonialisation by ''Schistosoma'' would take place rapidly. | ||
| + | |||
| + | The [http://www3.imperial.ac.uk/schisto '''Schistosomiasis Control Initiative'''] based at Imperial College is involved in treatment of this and six other NTD in sub-Saharan Africa. Professor Alan Fenwick and Dr Wendy Harrison have also been a great help in the development of our project (make sure to read up in our [https://2010.igem.org/Team:Imperial_College_London/Human_Practices/Report '''Human Practices Report''']). Alternatively here is a link to a [http://www.rockhopper.tv/programmes/207/ '''Documentary'''] about NTDs and their impact on human health, featuring Professor Alan Fenwick . | ||
| + | |||
| + | |||
| + | '''Prevention and Control - Detection of the parasite''' | ||
| + | |||
| + | The key to prevention of schistosomiasis is infrastructure, especially sanitation including water treatment and proper waste disposal. These relatively simple measures would not necessarily eliminate the parasite, but lower the case numbers significantly and restrict the infections more or less to people working in or near infected water. The key to success of these measures is that they break the parasites life cycle at the transmission stages between the host species. Whilst the implementation of modern sanitation would be a very desirable and hugely effective step to the prevention of schistosomiasis, it is also very expensive to provide whole communities with this kind of infrastructure, which on top of that would have to be maintained continuously to prevent relapses of the parasite. This is why health care organisations have taken a different approach, aiming to reduce morbidity rather than elimination of the parasite. Our project would be of great use to this strategy, as it would allow special mapping of ''Schistosoma'' presence, which could be followed up by targeted treatment of the population in the endemic areas. | ||
| + | So far methods to detect cercaria have been time consuming or require complex, spacious equipment. In the following section some previously developed trap designs are described. | ||
| + | |||
| + | [http://www.jstor.org/pss/3283499 Shiff ''et al.'' 1993] | ||
| + | |||
| + | [http://www.ajtmh.org/cgi/reprint/63/3/174.pdf Graczyk and Shiff 2000] | ||
| + | |||
| + | [http://www.ncbi.nlm.nih.gov/pubmed/12039676 Ahmed ''et al.'' 2002] | ||
| + | |||
| + | |||
| + | The traps make use of glass slides 25mm x 75mm coated with a matrix to which the cercaria adhere, such as agar or clear nail varnish, which proved to be most effective. This matrix is mixed with fatty acids, ranging from chemically pure linolic acid to locally produced seed oils, to acting as chemoattractant as well as stimulus for invasive behaviour that lures the cercaria to the trap. Once the attempt to burrow into the matrix they get stuck and can later be detected. In order to detect cercaria with this method, the whole trap has to be submerged for 2-3 hours. The second study analyses the use of this trap to detect avian ''Schistosoma'' which works, however with the same time constraints described before. the last study examines the use of locally produced oils, such as olive oil, which works to some extent, but not as efficiently as pure linoleic acid. This trap design therefore fails to provide results fast and additionally is not very reliable, particularly in flowing water. | ||
| + | |||
| + | A different approach to detection of cercaria is the use of mice. These are partially submerged in water for some time, usually around an hour, and, after some weeks for the parasite to developed, dissected. This method is both time-consuming and requires the killing of mice which makes it less than ideal for detection. | ||
| + | |||
| + | However there are now also a number of highly sophisticated techniques available, including the use of real time PCR for the detection of cercarial DNA sequences in the water, as well as high throughput sequencing of water samples. Whilst both of these are accurate and reliable methods, they require a great expertise and cannot be used in the field, causing delays in the risk analysis. | ||
| + | |||
| + | Therefore we think we have designed a great alternative to all the methods described above that allows fast and reliable detection of cercaria, under a variety of conditions, such as flowing or cloudy water. | ||
| + | |||
| + | '''Prevention and Control - Approaches to Vector control''' | ||
| + | |||
| + | [[Image:Snail collection bulinus biomphalaria oncomelania.JPG|thumb|right|200px|Picture of the following fresh water snails: ''Bulinus'', ''Biomphalaria'', ''Oncomelania'']] | ||
| + | |||
| + | In order to break the parasite life cycle many attempts have been made to control the parasite vector: The fresh water snails, in particular ''Biomphalaria'' snails, which transmits ''S. mansoni'', ''Oncomelani''a snails, which are the intermediate host of ''S. japonicum'', and ''Bulinus'' snails, giving rise to ''S. haematobium''. The methods attempting to reduce or even eradicate the vector in an area can be divided into three main categories: | ||
| + | |||
| + | '''Physical methods''' aim to disturb the snails’ habitat. As these snails are quite specialized to particular niches, changes in flora or displacement of the snails can result in their death. However this method is very work intensive, because each individual segment of lake or stream has to be treated individually. This type of approach has only been seriously tested in the USA to reduce avian ''Schistosoma'' in lakes, but has proven to be inefficient because the treated areas are quickly reclaimed by snails from adjacent sections of the lake or river. | ||
| + | |||
| + | '''Chemical methods''' attempt to use a wide variety of substances such as copper sulphate to kill the vectors. Whilst this can be more easily done on a large scale than the physical methods, they tend to have even more pronounced effects on the ecosystem, as they often kill many different species of molluscs as well as other creatures and diffuse to sections that have not been treated. Overall this method was used fairly successfully, although damage to the environment is considerable and the effects temporarily limited. | ||
| + | |||
| + | '''Biological methods''' are the last category of approaches. These, if done with great care, can be most environmentally friendly, although they equally have the potential to be the most devastating. The idea is to introduce a pathogen or predator, in some cases also a competitor for some resource, of the snails to be reduced. In the ideal case this will lead to the local extinction of the vector, however if the species supposed to reduce the vector is not chosen carefully it can become too wide spread, out-compete other species as well, or generally damage the ecosystem if too successful. There is always the risk of accidentally introducing an invasive species if this type of approach is taken. Furthermore the number of vectors is most likely to only be reduced, so the risk of infection would remain. | ||
| + | |||
| + | [[Image:Lake malawi2.JPG|centre|thumb|845px| Pictures of lake Malawi, a frequent source of ''Schistosoma'' infection for tourists. From the shore. From Space. ]] | ||
| + | |} | ||
| - | + | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:5px;" cellspacing="20" | |
| + | |style="font-family: helvetica, arial, sans-serif;font-size:2em;color:#ea8828;"|Fantastic Documentary about Schistosomiasis (AKA Bilharzia) | ||
| + | |- | ||
| + | |align="center"|<html><object width="571" height="366"><param name="movie" value="http://www.rockhopper.tv/flash/mxmlVideoPlayer.swf?id=18&src=http://www.rockhopper.tv/webservices/get-programme2.aspx&site=rockhopper"></param><param name="allowFullScreen" value="true"></param><param name="allowscriptaccess" value="always"></param><embed src="http://www.rockhopper.tv/flash/mxmlVideoPlayer.swf?id=18&src=http://www.rockhopper.tv/webservices/get-programme2.aspx&site=rockhopper" type="application/x-shockwave-flash" allowscriptaccess="always" allowfullscreen="true" width="571" height="366"></embed></object></html> | ||
| - | + | |} | |
Latest revision as of 23:22, 27 October 2010
| Schistosoma |
| Our system turns yellow when a Schistosoma parasite, which causes schistosomiasis or bilharzia, is present. It is the most devastating water-borne parasite in the world and causes huge problems in many developing countries. For more information, read on... |
| Schistosoma |
Schistosoma, from the class of trematodes constitute a genus commonly known as blood-flukes, are the parasites causing a disease called bilharzias or schistosomiasis which, according to the [http://www.who.int/mediacentre/factsheets/fs115/en/index.html WHO (2010)] is amongst the most devastating parasitic disease, second only to malaria, with 207 million infected and around 700 million at risk. The most significant species of Schistosoma are S. haematobium, S. mansoni, and S. japonicum, which combined cause a loss of over 1.5 million life years. Schistosomiasis shows typically a focal epidemiology and uniformly distributed amongst populations, with higher infection rates in children than in adults. Acute schistosomiasis, including symptoms such as fever and malaise, is most commonly found in primary infected individuals whereas chronic schistosomiasis and its accompanying complications such as hepatic or intestinal schistosomiasis, are more predominant in people with long lasting infection mainly in poor rural areas. This is caused by a slow process of immune acquisition as well as the long lasting effect of schistosomal eggs trapped in tissues leading to inflammatory reactions. Diagnosis is usually accomplished by microscopy of stool samples although in many cases, especially in rural settings, treatment with Praziquantel is done without individual diagnosis. Much progress has been made in the control of the disease through community-based chemotherapy however these rely on political commitment and strong health systems. To understand the disease itself, as well as the possible approaches to improving its control we have to gain a deeper understanding of the complex life cycle of this parasite. Like many other eukaryotic parasites, the Schistosoma life cycle involving several stages in and outside two different hosts.
Infected people can pass out several thousands of parasite eggs in their stool (most species) or urine (Schistosoma haematobium). These eggs were first discovered in Egypt by Theodor Maximilian Bilharz, a German pathologist. Under the microscope one can see that they are around 110-170 µm long by 40 to 70 µm wide giving them an elongated shape with a distinctive terminal spine that is used for identification. These characteristically shaped eggs are viable for around week and once in contact with water they quickly hatch and release the first larval form called miracidia. These free-swimming, ciliated larvae cannot feed and will usually die within 24 hours if they fail to detect a suitable intermediary host, using light and chemical stimuli, and subsequently enter it. Different Schistosoma species show preference for different host species but at this stage all rely on fresh water snails. Once inside the host the miracidia reproduce asexually and form multicellular sporocysts which later develop into cercarial larvae with embryonic suckers and a characteristic bifurcated tail. This process takes between 4 and 6 weeks from which time on thousands of cercaria, a second larval form, can be shed from the snails daily for several month. This shedding is induced by light [http://www.ncbi.nlm.nih.gov/pubmed/16997665 (Gryseels et al. 2006)] and aims to maximise the likelihood of cercaria finding their second host. The larvae can survive in the water for up to 72 hours, although more commonly around 30 hours, during which time they use chemical stimuli as well as light and temperature to detect the skin of a suitable host. By far the most powerful stimulus to triggering invasive behaviour are skin lipids, in particular medium-chain free fatty acids [http://www.ncbi.nlm.nih.gov/pubmed/11983589 (McKerrow and Salter 2002)]: Of the C18 fatty acids examined, stearic (18:0) is inactive, oleic (18:1) slightly active, linoleic (18:2) and linolenic (18:3) acids highly active [http://journals.cambridge.org/action/displayAbstract;jsessionid=B1A531CD8298B78F36E5A5A336522AD3.tomcat1?fromPage=online&aid=4180708 (Austin et al. 1974)]. Once detected by the parasite these stimuli lead to signal-dependent breakdown of inositol phospholipids which is directly linked to activation of protein kinase C (via elevated diacylglycerol level) and mobilization of calcium (via elevated levels of inositol triphosphate) which in turn evokes subsequent cellular response [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WFH-4C52GG2-FP&_user=217827&_coverDate=04%2F30%2F1991&_alid=1505009318&_rdoc=1&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=6795&_sort=r&_st=13&_docanchor=&view=c&_ct=4&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=b5b1e3b6d044a907895dc267d542cf09&searchtype=a (Matsumura et al. 1991)] such as the release of enzymes from a specialized gland - called acetabular gland complex – at the posterior region of the head of the parasite [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WFH-4C52GPD-KH&_user=217827&_coverDate=08%2F31%2F1992&_alid=1505013773&_rdoc=2&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=6795&_sort=r&_st=13&_docanchor=&view=c&_ct=8&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=273e73fe67245cd2feb84a18cc1fbbec&searchtype=a (Fishelson et al. 1992)]. These enzymes include number of proteases that target proteins in the host's skin such as keratin and elastin the latter of which is cleaved by the most essential and abundant protease called schistosomal elastase, which we use in our iGEM project to detect Schistosoma. This process allows cercaria to burrow through the skin of their new host, where they leave their tail behind, into blood vessels. There are six species of Schistosoma known to successfully infect humans: Schistosoma mansoni , which uses Biomphalaria snails as intermediate host and can cause intestinal and hepatic schistosomiasis in Africa, the Arabian peninsula as well as South America; S. japonicum, a zoonotic parasite, which uses Oncomelania snails as intermediate host and can cause intestinal and hepatosplenic schistosomiasis, especially in South-East Asia; S. mekongi, S. intercalatum and related S. guineansis ,which are restricted to small areas an only of local importance; as well as S. haematobium, transmitted by Bulinus snails, which can give rise to urinary schistosomiasis in Africa and the Arabian peninsula Additionally there is number of avian Schistosoma species, especially in Northern Europe, the USA and Canada that cannot infect humans but will still try to enter our skin giving rise to the harmless but unpleasant condition called swimmer's itch. However these conditions, like cercarial dermatitis often go unnoticed in endemic areas. Once inside the human body, the cercaria travel through the blood via the lungs into the portal vein of the liver where they develop into schistosomulae. After a maturation period one to one and a half month after which they mate. The way Schistosoma have evolved to mate is unique amongst the trematodes in that there is a significant sexual dimorphism, which also inspired their name which translates from Greek to 'split body'. This name describes the male anatomy as there is a long groove, called gynecophoral canal, along its body in which the female is held. Adult males are between 10 to 15 mm long whereas females are longer, between 16 to 22mm, and thinner. Both sexes have two suckers – one on each end – to hold onto the walls of blood vessels where they feed on blood and globulins through anaerobic glycolysis. The debris is regurgitated in the host’s blood. After mating they migrate to their perivesicular (S. haematobium) or mesenteric (other species) destination where the female releases her eggs. With an average life span of 3 to 5 years, although up to 30 years is possible, The theoretical reproduction potential of one schistosome pair is up to 600 billion schistosomes [http://www.ncbi.nlm.nih.gov/pubmed/16997665 (Gryseels et al. 2006)]. 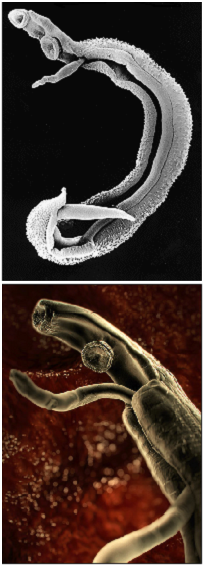 Pictures of Schistosoma. Top: Electron microscopy of Schistosoma couple [http://observationsofanerd.blogspot.com/2009/06/this-weeks-sci-fi-worthy-parasite.html Link]. Bottom: Artistic impression of schistosome parasite in blood vessel [http://animal.discovery.com/invertebrates/monsters-inside-me/schistosomiasis-schistosoma-mansoni/ Link]. The eggs themselves secrete proteolytic enzymes that allow the eggs to reach their destination: the bladder (S. Haematobium) or intestines (other species) from which they are released by the host into the environment, allowing the cycle to continue.
The first signs of infection can often be observed directly after penetration of the skin causing a temporary rash that can persist for days as paulopruriginous lesions. This occurs in particular after primary infection with the parasite frequently found in migrants into and tourists returning from endemic areas as a study by [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WJT-4H4T0M2-3&_user=217827&_coverDate=05%2F31%2F2006&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=e7f6675f5954192c9e6b1c1b64d6a484&searchtype=a Bottieau et al. 2006 ] showed. The more progressive stages of schistosomiasis, often referred to as Katayama fever, are also rarely diagnosed in the endemic population, maybe as a result of under diagnosis, but more commonly found in migrants and tourists also. The trained immune system of healthy people living in endemic areas will often be able to fight off parasite infections naturally. Furthermore cord blood lymphocytes from newborns of schistosome-infected mothers are significantly more likely to amount a helminth Ag-driven B cell response than others, indicating in utero sensitisation takes place [http://www.jimmunol.org/cgi/content/full/160/7/3578 (King et al. 1998)]. Acute schistosomiasis is a systemic hypersensitivity reaction caused by the migration of schistosomulae within the host body and usually weeks or even months after primary infection. It is characterised by a sudden onset of symptoms ranging from fever, fatigue, and myalgia over malaise, non-productive cough, eosinophilia and patchy infiltrates on chest radiography. These can later also be accompanied by abdominal symptoms caused by movement of mature worms.
Chronic pathology caused by schistosome infection is rarely evoked by the adult worms, but rather by lesion caused by the eggs that become trapped in the tissue during perivesical or perintestinal migration, or in other cases after embolisation by various organs including the liver, spleen, lung and cerebrospinal system. As a result of protease secretion, an eosinophilic inflammatory response and granulomatous reaction are provoked which are later replaced by fibrous deposits. The granulomatous responses are "strictly mediated by CD4+ T helper (Th) cells specific for egg antigens" [http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0036-46652005000400001&lng=en&nrm=iso&tlng=en Nascimento-Carvalho & Moreno-Carvalho 2005]. The extent to which an individual suffers is therefore directly dependent on the disease burden but also the precise immune response mounted by the individual. Depending on the species of schistosome a person is infected with, a number of distinct conditions can be found, if the disease enters the chronic stages and is not treated timely. In addition to the immunopathology that can be attributed directly to the parasite, it is although thought to be a major factor contributing to morbidity and accompanying problems such as anaemia, nutritional status and cognitive and physiological capacity, as well as fatigue [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T1B-4G2BRY1-12&_user=217827&_coverDate=05%2F06%2F2005&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_searchStrId=1510251595&_rerunOrigin=google&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=505520bc94a7d520db8ae3abeb1033b1&searchtype=a (King et al. 2005)]. Older studies failed to demonstrate this effect even in individuals, but more recent studies have shown a small, yet significant, effect of the parasite on human health in general. Urinary schistosomiasis This complication caused by Schistosoma is most notably found in young children between 5 and 10 and is rare during and after adolescence. The eggs of S. haematobium, which are excreted via urine as part of the parasite life cycle, can be trapped in the genitourinal system and elicit a granulomatous inflammation, ulceration or pseudopolyposis both in adjacent blood vessels and the urethral walls. The early symptoms include dysuria, pollakisuria, proteinuria and most importantly haematuria. The later can vary from small amount of blood found in the terminal urine to darker colouration of the whole urine sample in more severe cases and the condition can often be exacerbate by bacterial superinfections or bladder stones. The proportion of untreated infected individuals with bladder complication to those without seems to vary significantly (between 0% and 97%) however after treatment with Praziquantel a full recovery is made in most cases. Notably this suggests that the renal parenchyma is usually compressed but not destroyed although schistosomiasis-induced renal damage can be fatal particularly for older patients [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T1B-4KY3RPD-M&_user=217827&_coverDate=09%2F29%2F2006&_alid=1505007608&_rdoc=5&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=4886&_sort=r&_st=13&_docanchor=&view=c&_ct=9&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=27498d6154512f9cccda7ff0d2fbb805&searchtype=a (Gryseels et al. 2006)]. Furthermore a study by [http://www.nature.com/search/executeSearch?sp-q-1=BJC&sp-q=Schistosomiasis+and+the+risk+of+bladder+cancer+in+Alexandria%2C+Egypt&sp-c=25&sp-m=0&sp-s=date_descending&include-collections=journals_nature%2Ccrawled_content&exclude-collections=journals_palgrave%2Clab_animal&sp-a=sp1001702d&sp-sfvl-field=subject|ujournal&sp-x-1=ujournal&sp-p-1=phrase&sp-p=all&submit=go Bedwani et al. 1998] also found urinary schistosomiasis to be a significant contributor to bladder cancer accounting for around 16% of the cases amongst the Egyptian population and the [http://monographs.iarc.fr/ENG/Monographs/vol61/volume61.pdf IARC (1997)] found that there is sufficient evidence for the carcinogenicity of infection with S. haematobium. Intestinal schistosomiasis Once again the eggs of the parasite induce an immune response in the form of mucosal granulomatous inflammation, pseudopolyposis, microulcerations, and superficial bleeding, with lesions mostly located in the large bowel and rectum. Symptoms include chronic as well as intermittent abdominal pain and discomfort, loss of appetite, diarrhoea, and bloody faeces. This condition manifests itself in chronically infected individuals at a relatively moderate level between 3-55% with 30-60% of diarrhoea cases attributable to Schistosoma infection. However the statistical significance of these numbers is debatable as sampling techniques have varied across different studies. Hepatic schistosomiasis This is one of the most significant complications Schistosoma can cause during chronic infection, which can cause long-lasting, potentially fatal health problems, even after treatment with Praziquantel. It is caused by S. mansoni, S. japonicum and S. mekongi. Technically early inflammatory and late fibriotic hepatic disease, also called Symmers' clay-pipestem fibrosis of the liver, are two distinct conditions, in terms of morbidity, clinical practise and immunological mechanism, although they are combined here as hepatic (and hepatosplenic) schistosomiasis [http://www.ajtmh.org/cgi/content/abstract/17/1/38 Cheever (1968)]. A mild inflammation is most common in young individuals especially during the early stages of infection and can be observed in around 80% of infected children. The main cause of hepatomegaly are ova which got caught in the presinusoidal periportal spaces of the liver, giving rise to sharp-edged enlargements of the left liver lobe as well as nodular splenomegaly reaching from the costal arch to the umbilicus or in extreme cases even to the pelvis. During chronic infection with Schistosoma however fibrotic or chronic hepatic schistosomiasis can arise in a small proportion of mostly young to middle aged adults in particular those with a high parasite load, although immunogenetic predispositions are thought to be the most significant risk factor [http://www.jimmunol.org/cgi/search?sortspec=relevance&author1=Chevillard&fulltext=schistosomiasis&pubdate_year=2003&volume=&firstpage= (Chevillard et al. 2003)]. The disease is caused by heavy deposition of diffuse collagen in the periportal space leading to a hardening of the liver and obstruction of blood flow through the portal veins, which can result in portal hypertension, splenomegaly, and others including commonly fatal bleedings in that latest stages [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B75J9-4G7WVDX-7&_user=217827&_coverDate=09%2F01%2F2000&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_searchStrId=1509470188&_rerunOrigin=scholar.google&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=a872e3647a4e62a95325c92be7470219&searchtype=a (Bica et al. 2000)]. The liver complications caused by S. japonicum can progress much faster from early mild to chronic stages resulting in sudden onset of bleeding. Neuroschistosomiasis This condition is the second most common presentation of infection by S. mansoni also causing granulomatous inflammatory reaction due to schistosome eggs reaching the spinal cord or brain via the vascular system, mainly through "arterial or retrograde venous blood flow via the valveless perivertebral plexus of Batson", or by occasional migration of adult schistosomes. The main clinical syndromes are spinal cord neuroschistosomiasis divided into acute or subacute myelopathy and localized cerebral or cerebella neuroschistosomiasis. The later gives rise to focal CNS impairment, seizures as well as increased intracranial pressure. Presumptive diagnosis of neuroschistosomiasis relies on confirmation of the presence of S. mansoni infection by stool microscopy for trematode eggs, and also serologic testing of blood and spinal fluid. Because this condition is relatively rare in Western countries, it is often hard to diagnose. It should be considered if a patient has a history of exposure to schistosome-infected water and shows any of the following symptoms: seizure, ataxia, increased intracranial pressure, hemianopsia, nystagmus and vertigo or paraplegia, sphincter dysfunction, and sensory disturbances from the pelvic girdle down, as these are the neurological effects most commonly brought about neuroschistosomiasis. Other frequent complaints are leg weakness, bladder dysfunction and low back pain [http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0036-46652005000400001&lng=en&nrm=iso&tlng=en Nascimento-Carvalho & Moreno-Carvalho 2005]. Ectopic schistosomiasis Schistosomiasis can less frequently also cause complications in other organs, such as lung or the genitalia. These as well are caused by the parasite eggs and the ensuing inflammatory reaction. Lesions in the genital areas such as vulva and vagina might have implications for sexual transmission, although this is not well documented. Additionally there is the risk of infertility in those cases where lesions and inflammations occur near the testicles or the fallopian tube and ovaries.
There are a number of drugs available to cure infection with schistosomes, for example amoscanate, chloroxylenol, meclonazepam and oxamniquine. However the by far most widely used helminticide to combat schistosomiasis is Praziquantel despite not being licensed for medical treatment of humans in the UK. It is effective against Schistosoma in a single treatment, although it should be followed up by a second treatment after 4-6 weeks, and is also used for some other parasitic trematodes such as liver flukes. Although the drug has been in use for over 20 years, the mechanism of its action is still not understood [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T29-4V1KMJN-1&_user=217827&_coverDate=03%2F31%2F2009&_rdoc=1&_fmt=high&_orig=search&_origin=search&_sort=d&_docanchor=&view=c&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=d2d7b0f7be37d4568b69a5b61438f7d8&searchtype=a (Aragon et al. 2009)]. Side effects are usually mild, although in cases with high parasite load they can be more severe as a result of massive release of parasitic antigens from the dead worms. "Praziquantel is the recommended treatment for schistosomiasis at 40 mg/kg body weight. The cost of a single 600-mg tablets is about US$ 0.08 and an average treatment is estimated to be between US$ 0.20–0.30. Praziquantel is now available free of charge to a few high-disease burden least developed countries (LDC), through a donation from Merck KGaA to the World Health Organization." [http://www.who.int/schistosomiasis/strategy/en/index.html (WHO, 2010)]
Because of the difficult nature of schistosomiasis diagnosis, treatment is often not provided after individual diagnosis, but instead it is very common to test only a sample of individuals in a community, especially school children, and if a certain proportion of the sampled people is infected, the whole community is treated with Praziquantel. This measure allows control of the disease to some extend if done in a coordinated approach with treatment on a large scale with safe and effective drug, and at regular intervals [http://www.who.int/schistosomiasis/strategy/en/index.html (WHO, 2010)]. The communal approach aims at reducing the late stages of schistosomiasis that go along with malnutrition and often cause developmental problem in children. Based on the sampled disease burden is also the length of the period after which treatment is repeated. This method will not eradicate the disease but rather to lower the total number of infected people and prevent severe infection in order to reduce morbidity as well as socio-economic impact. In theory it is also possible to break the parasitic life cycle with this method and permanently prevent reinfection, however in practise it is well known that eradication does not take place for a number of different reasons, which is why treatment is repeated regularly. Even if Schistosoma were completely eradicated from one area, due to the continuing presence of the water snail vector, recolonialisation by Schistosoma would take place rapidly. The [http://www3.imperial.ac.uk/schisto Schistosomiasis Control Initiative] based at Imperial College is involved in treatment of this and six other NTD in sub-Saharan Africa. Professor Alan Fenwick and Dr Wendy Harrison have also been a great help in the development of our project (make sure to read up in our Human Practices Report). Alternatively here is a link to a [http://www.rockhopper.tv/programmes/207/ Documentary] about NTDs and their impact on human health, featuring Professor Alan Fenwick .
The key to prevention of schistosomiasis is infrastructure, especially sanitation including water treatment and proper waste disposal. These relatively simple measures would not necessarily eliminate the parasite, but lower the case numbers significantly and restrict the infections more or less to people working in or near infected water. The key to success of these measures is that they break the parasites life cycle at the transmission stages between the host species. Whilst the implementation of modern sanitation would be a very desirable and hugely effective step to the prevention of schistosomiasis, it is also very expensive to provide whole communities with this kind of infrastructure, which on top of that would have to be maintained continuously to prevent relapses of the parasite. This is why health care organisations have taken a different approach, aiming to reduce morbidity rather than elimination of the parasite. Our project would be of great use to this strategy, as it would allow special mapping of Schistosoma presence, which could be followed up by targeted treatment of the population in the endemic areas. So far methods to detect cercaria have been time consuming or require complex, spacious equipment. In the following section some previously developed trap designs are described. [http://www.jstor.org/pss/3283499 Shiff et al. 1993] [http://www.ajtmh.org/cgi/reprint/63/3/174.pdf Graczyk and Shiff 2000] [http://www.ncbi.nlm.nih.gov/pubmed/12039676 Ahmed et al. 2002]
A different approach to detection of cercaria is the use of mice. These are partially submerged in water for some time, usually around an hour, and, after some weeks for the parasite to developed, dissected. This method is both time-consuming and requires the killing of mice which makes it less than ideal for detection. However there are now also a number of highly sophisticated techniques available, including the use of real time PCR for the detection of cercarial DNA sequences in the water, as well as high throughput sequencing of water samples. Whilst both of these are accurate and reliable methods, they require a great expertise and cannot be used in the field, causing delays in the risk analysis. Therefore we think we have designed a great alternative to all the methods described above that allows fast and reliable detection of cercaria, under a variety of conditions, such as flowing or cloudy water. Prevention and Control - Approaches to Vector control In order to break the parasite life cycle many attempts have been made to control the parasite vector: The fresh water snails, in particular Biomphalaria snails, which transmits S. mansoni, Oncomelania snails, which are the intermediate host of S. japonicum, and Bulinus snails, giving rise to S. haematobium. The methods attempting to reduce or even eradicate the vector in an area can be divided into three main categories: Physical methods aim to disturb the snails’ habitat. As these snails are quite specialized to particular niches, changes in flora or displacement of the snails can result in their death. However this method is very work intensive, because each individual segment of lake or stream has to be treated individually. This type of approach has only been seriously tested in the USA to reduce avian Schistosoma in lakes, but has proven to be inefficient because the treated areas are quickly reclaimed by snails from adjacent sections of the lake or river. Chemical methods attempt to use a wide variety of substances such as copper sulphate to kill the vectors. Whilst this can be more easily done on a large scale than the physical methods, they tend to have even more pronounced effects on the ecosystem, as they often kill many different species of molluscs as well as other creatures and diffuse to sections that have not been treated. Overall this method was used fairly successfully, although damage to the environment is considerable and the effects temporarily limited. Biological methods are the last category of approaches. These, if done with great care, can be most environmentally friendly, although they equally have the potential to be the most devastating. The idea is to introduce a pathogen or predator, in some cases also a competitor for some resource, of the snails to be reduced. In the ideal case this will lead to the local extinction of the vector, however if the species supposed to reduce the vector is not chosen carefully it can become too wide spread, out-compete other species as well, or generally damage the ecosystem if too successful. There is always the risk of accidentally introducing an invasive species if this type of approach is taken. Furthermore the number of vectors is most likely to only be reduced, so the risk of infection would remain. |
| Fantastic Documentary about Schistosomiasis (AKA Bilharzia) |
 "
"