Team:Newcastle/DNA re-hydration
From 2010.igem.org
(Difference between revisions)
Shethharsh08 (Talk | contribs) (→DNA Re-hydration) |
(→Method) |
||
| (14 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Team:Newcastle/mainbanner}} | {{Team:Newcastle/mainbanner}} | ||
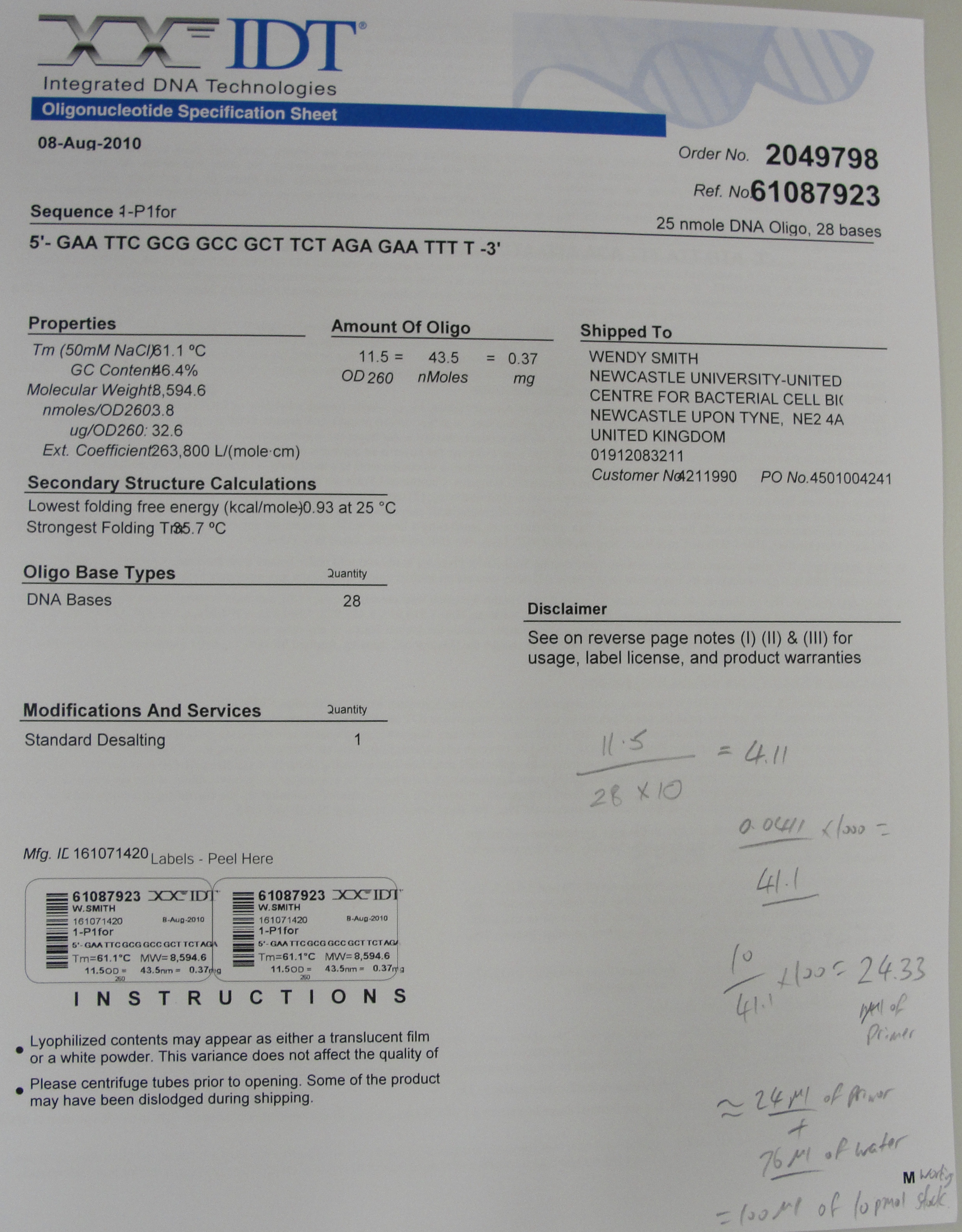
| - | [[Image:Newcastle OSS.JPG|right|200px|Oligonucleotide Specification Sheet (OSS) with the calculations used to work out the volumes of rehydrated primer and H2O required for this specific primer]] | + | [[Image:Newcastle OSS.JPG|right|200px| Oligonucleotide Specification Sheet (OSS) with the calculations used to work out the volumes of rehydrated primer and H2O required for this specific primer]] |
=DNA Re-hydration= | =DNA Re-hydration= | ||
| Line 9: | Line 9: | ||
* Oligonucleotide Specification Sheet (OSS) | * Oligonucleotide Specification Sheet (OSS) | ||
| - | * | + | * OD260 values |
| - | * | + | * Sterile Pure Lab Distilled Water |
| - | + | ||
| - | + | ||
| - | == | + | ==Method== |
| - | # To begin, read the Oligonucleotide Specification Sheet to find the | + | # To begin, read the Oligonucleotide Specification Sheet to find the OD260 values and number of DNA bases for each primer/DNA. |
| - | # Once those values are found, divide the ( | + | # Once those values are found, divide the (OD260 value) by (number of DNA bases x10). |
# Multiple the result by 1000 | # Multiple the result by 1000 | ||
| - | # Divide 10 by the previous result, and multiple all by 100: the end result is the volume of primer | + | # Divide 10 by the previous result, and multiple all by 100: the end result is the volume of primer DNA (in µl) required to prepare 100µl at 10pM concentration. |
| - | # | + | # Rehydrate lyopholsed primers in 1ml sterile water |
| - | + | # Remove the appropiate volume of rehydrated primer and add Pure Lab Distilled Water to make the solution up to 100 µl in total. | |
Please note: | Please note: | ||
| Line 28: | Line 26: | ||
| - | |||
[[Image:Newcastle Pure Lab Flex 2.JPG|200px]] | [[Image:Newcastle Pure Lab Flex 2.JPG|200px]] | ||
| + | |||
| + | '''Figure 1''' showing Pure Lab Distilled Water apparatus. | ||
| + | |||
| + | '''Go back to our [[Team:Newcastle/Protocol list|Protocol List]]''' | ||
{{Team:Newcastle/footer}} | {{Team:Newcastle/footer}} | ||
Latest revision as of 23:21, 27 October 2010

| |||||||||||||
| |||||||||||||
DNA Re-hydration
This protocol is used for re-hydration of DNA. We used this mainly for our newly arrived primers.
Materials required
- Oligonucleotide Specification Sheet (OSS)
- OD260 values
- Sterile Pure Lab Distilled Water
Method
- To begin, read the Oligonucleotide Specification Sheet to find the OD260 values and number of DNA bases for each primer/DNA.
- Once those values are found, divide the (OD260 value) by (number of DNA bases x10).
- Multiple the result by 1000
- Divide 10 by the previous result, and multiple all by 100: the end result is the volume of primer DNA (in µl) required to prepare 100µl at 10pM concentration.
- Rehydrate lyopholsed primers in 1ml sterile water
- Remove the appropiate volume of rehydrated primer and add Pure Lab Distilled Water to make the solution up to 100 µl in total.
Please note:
- Extra care must be taken when following this protocol, i.e. there must be NO contamination.
- The newly arrived primer tubes have to be handled with extra caution, because they will be the main stocks which working stock solutions will be made from. Therefore, gloves have to be worn, as well as preventing any contamination. Water will be used in order to liquefy the primers and the water used will be from Pure Lab Distilled Water.
Figure 1 showing Pure Lab Distilled Water apparatus.
Go back to our Protocol List
 
|
 "
"