Team:Newcastle/23 July 2010
From 2010.igem.org
Shethharsh08 (Talk | contribs) |
RachelBoyd (Talk | contribs) (→Conclusion) |
||
| (38 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | + | {{Team:Newcastle/mainbanner}} | |
| - | + | ||
| - | == | + | =Arginine experiment= |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | == | + | ==Aim== |
| + | The aim of this experiment is to determine whether ''B. subtilis'' 168 is able to take up external arginine. | ||
| - | == | + | ==Procedure== |
| - | + | * Please refer to [[Team:Newcastle/Arginine test| Arginine test]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | == | + | ==Results== |
| - | + | Arginine is an amino acid that is positively charged. Therefore if ''B. subtilis'' 168 is able to take up arginine, it will cause a pH change in the media. This would result in an increase in pH. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | |[[Image:Newcastle_arginine_test_230710.png|500px ]] | |
| - | + | ||
| - | + | ||
| - | + | ||
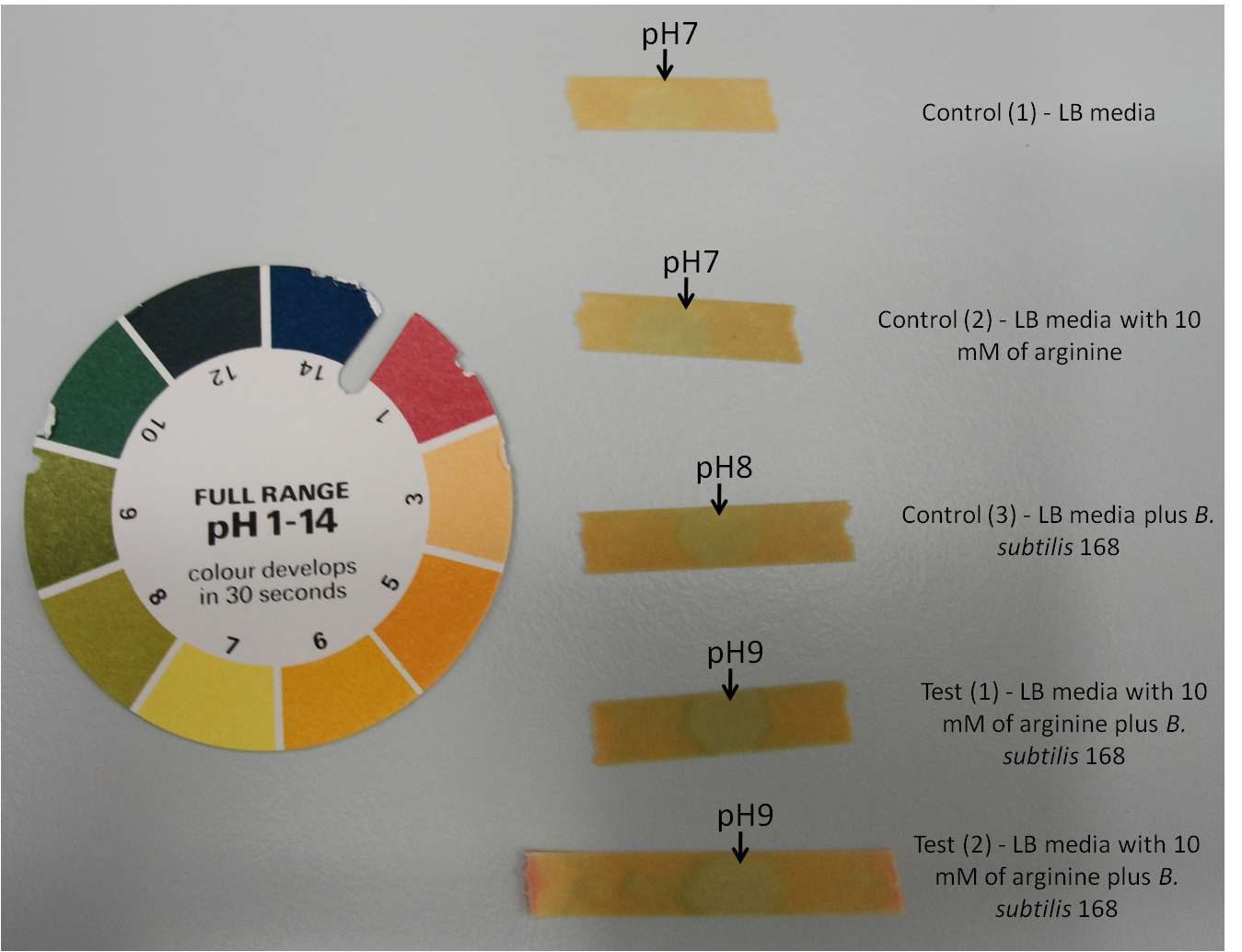
| - | + | '''Figure 1:''' Arginine test using pH indicator stick to measure pH changes of the media. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | === | + | {|border=1 |
| - | + | |- | |
| - | + | !Time (in minutes) | |
| - | + | !Control (1) | |
| + | !Control (2) | ||
| + | !Control (3) | ||
| + | !Test (1) | ||
| + | !Test (2) | ||
| + | |- | ||
| + | |0 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |- | ||
| + | |30 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |- | ||
| + | |60 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |- | ||
| + | |90 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |- | ||
| + | |120 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 8 | ||
| + | |pH 8 | ||
| + | |- | ||
| + | |150 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 8 | ||
| + | |pH 8 | ||
| + | |- | ||
| + | |180 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 9 | ||
| + | |pH 9 | ||
| + | |- | ||
| + | |210 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 9 | ||
| + | |pH 9 | ||
| + | |- | ||
| + | |240 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 9 | ||
| + | |pH 9 | ||
| + | |- | ||
| + | |270 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 8 | ||
| + | |pH 9 | ||
| + | |pH 9 | ||
| + | |- | ||
| + | |300 | ||
| + | |pH 7 | ||
| + | |pH 7 | ||
| + | |pH 8 | ||
| + | |pH 9 | ||
| + | |pH 9 | ||
| + | |} | ||
| + | |||
| + | '''Table 1:''' Arginine test using pH indicator stick to measure pH changes of the media. Table represents the change in pH over the time span of 300 minutes i.e. 5 hours. | ||
| + | |||
| + | Here, | ||
| + | #Control (1) - LB media | ||
| + | #Control (2) - LB media with 10 mM of arginine | ||
| + | #Control (3) - LB media plus ''B. subtilis'' 168 | ||
| + | #Test (1) - LB media with 10 mM of arginine plus ''B. subtilis'' 168 | ||
| + | #Test (2) - LB media with 10 mM of arginine plus ''B. subtilis'' 168 | ||
| + | |||
| + | ==Conclusion== | ||
| + | ''B. subtilis'' 168 breaks down arginine to urea by producing arginase. The urea is then further broken down to ammonia and carbonate ions by urease. This will lead to an increase in pH. Both the test 1 and test 2, which contain ''B. subtilis'' 168 and 10 mM of arginine show an increase in pH from 7 to 9. While the control 1 and control 2, which contain no ''B. subtilis'' 168 remains at pH 7. The control 3 which contain ''B. subtilis'' 168 but without addition of arginine show an increase in pH from 7 to 8. This could be due to unidentified products that are secreted by the bacteria. | ||
| + | |||
| + | Therefore this experiment indicates that ''B. subtilis'' 168 is able to utilise arginine, and thus increase the overall pH of the media. The production of carbonate ion will lead to its binding with calcium ions provided in the media leading to formation of calcium carbonate. | ||
| + | |||
| + | {{Team:Newcastle/footer}} | ||
Latest revision as of 21:00, 27 October 2010

| |||||||||||||
| |||||||||||||
Contents |
Arginine experiment
Aim
The aim of this experiment is to determine whether B. subtilis 168 is able to take up external arginine.
Procedure
- Please refer to Arginine test
Results
Arginine is an amino acid that is positively charged. Therefore if B. subtilis 168 is able to take up arginine, it will cause a pH change in the media. This would result in an increase in pH.
Figure 1: Arginine test using pH indicator stick to measure pH changes of the media.
| Time (in minutes) | Control (1) | Control (2) | Control (3) | Test (1) | Test (2) |
|---|---|---|---|---|---|
| 0 | pH 7 | pH 7 | pH 7 | pH 7 | pH 7 |
| 30 | pH 7 | pH 7 | pH 7 | pH 7 | pH 7 |
| 60 | pH 7 | pH 7 | pH 7 | pH 7 | pH 7 |
| 90 | pH 7 | pH 7 | pH 7 | pH 7 | pH 7 |
| 120 | pH 7 | pH 7 | pH 7 | pH 8 | pH 8 |
| 150 | pH 7 | pH 7 | pH 7 | pH 8 | pH 8 |
| 180 | pH 7 | pH 7 | pH 7 | pH 9 | pH 9 |
| 210 | pH 7 | pH 7 | pH 7 | pH 9 | pH 9 |
| 240 | pH 7 | pH 7 | pH 7 | pH 9 | pH 9 |
| 270 | pH 7 | pH 7 | pH 8 | pH 9 | pH 9 |
| 300 | pH 7 | pH 7 | pH 8 | pH 9 | pH 9 |
Table 1: Arginine test using pH indicator stick to measure pH changes of the media. Table represents the change in pH over the time span of 300 minutes i.e. 5 hours.
Here,
- Control (1) - LB media
- Control (2) - LB media with 10 mM of arginine
- Control (3) - LB media plus B. subtilis 168
- Test (1) - LB media with 10 mM of arginine plus B. subtilis 168
- Test (2) - LB media with 10 mM of arginine plus B. subtilis 168
Conclusion
B. subtilis 168 breaks down arginine to urea by producing arginase. The urea is then further broken down to ammonia and carbonate ions by urease. This will lead to an increase in pH. Both the test 1 and test 2, which contain B. subtilis 168 and 10 mM of arginine show an increase in pH from 7 to 9. While the control 1 and control 2, which contain no B. subtilis 168 remains at pH 7. The control 3 which contain B. subtilis 168 but without addition of arginine show an increase in pH from 7 to 8. This could be due to unidentified products that are secreted by the bacteria.
Therefore this experiment indicates that B. subtilis 168 is able to utilise arginine, and thus increase the overall pH of the media. The production of carbonate ion will lead to its binding with calcium ions provided in the media leading to formation of calcium carbonate.
 
|
 "
"