Team:Newcastle/22 June 2010
From 2010.igem.org
RachelBoyd (Talk | contribs) (→Set up ligation) |
RachelBoyd (Talk | contribs) |
||
| Line 1: | Line 1: | ||
{{Team:Newcastle/mainbanner}} | {{Team:Newcastle/mainbanner}} | ||
'''Tuesday''' | '''Tuesday''' | ||
| + | ===Aims=== | ||
| + | The aim of today's Lab practice was to extract the plasmids for GFP and RFP, digest the 2 plasmids and extract the 2 inserts and 1 of the backbones (vector)from a gel. From this a ligation was set up. | ||
| + | |||
| + | ===Equipement List=== | ||
| + | |||
| + | For today's Lab pratice we required: | ||
| + | |||
| + | * Broth cultures from yesterday | ||
| + | * Solutions for Minipreps( plasmid and gel extraction,digest and ligation) | ||
| + | * Buffers | ||
| + | * Agarose gel | ||
| + | * Microwave | ||
| + | * Restriction enzymes | ||
| + | * Gloves(for handling the gel) | ||
| + | * Scalpel | ||
| + | * Scales | ||
| + | * Transilluminator | ||
| + | * Nanodrop | ||
| + | |||
| + | |||
===Qiagen miniprep: Plasmid extraction=== | ===Qiagen miniprep: Plasmid extraction=== | ||
The 5ml culture set up yesterday was pelleted down using the microcentrifuge, the pellet was resuspended in buffer P1. | The 5ml culture set up yesterday was pelleted down using the microcentrifuge, the pellet was resuspended in buffer P1. | ||
Revision as of 09:22, 12 July 2010

| |||||||||||||
| |||||||||||||
Tuesday
Contents |
Aims
The aim of today's Lab practice was to extract the plasmids for GFP and RFP, digest the 2 plasmids and extract the 2 inserts and 1 of the backbones (vector)from a gel. From this a ligation was set up.
Equipement List
For today's Lab pratice we required:
- Broth cultures from yesterday
- Solutions for Minipreps( plasmid and gel extraction,digest and ligation)
- Buffers
- Agarose gel
- Microwave
- Restriction enzymes
- Gloves(for handling the gel)
- Scalpel
- Scales
- Transilluminator
- Nanodrop
Qiagen miniprep: Plasmid extraction
The 5ml culture set up yesterday was pelleted down using the microcentrifuge, the pellet was resuspended in buffer P1. Lysis buffer P2 was used to release the DNA from the cell (this buffer contains RNAse to help reduce RNA contamination). PE buffer containing ethanol was used as a wash. We lysed the cells for 1 minute, gently inverting the tube. We neutalised the lysis with N3 buffer and centrifuged for 10minutes, only the plasmid DNA was left in suspension. The DNA was eluted at low salt concentration through a column membrane.
Digest
Cut with restriction enzyme EcoR1 and Pst1 1 μl of each. 10*buffer ... so 3 μl in 30μ1 therefore we can have 25μ1 of DNA Also no more than 10% Glycerol in the enzyme solution or the reaction would be inhibited.
Gel extraction
Agarose gel
Agarose gel is used for DNA seperation. 1% agarose is used because it is suitable for most kilobase pairs of DNA. We used 60ml for our gel. We used SafeView dye to bind the DNA so that the DNA is visible. We used TAE buffer and boiled the mixture in a microwave to melt it. We then let it cool and poured it into the tank to set.
Electrophoresis
In lane one we loaded the molecular marker 5μl. In lane three we loaded the digested RFP plasmid. In lane five we loaded the digested GFP plasmid. In lane eight we loaded 5μl RFP plasmid and 1μl sample buffer. Lanes two, four, six and seven are empty.
Cut gel out
We cut the inserts which were about 900bp and we use the backbone from the RFP plasmid.
QIAquick Gel extraction kit
The DNA fragments are cut from the agarose gel with a scalpel. Three parts of QG buffer is added to one part of gel and the mixtures are dissolved at the temperature of 50°C. The tubes are vortexed every 2-3 minutes to help dissolve the gel. 1 part of isopropanol is added to the sample and mix. The microcubes were spinned in the microfuge for 1 minute.
Weighing
All gel extractions weighed 0.3g.
Set up ligation
| Reagents | 1:3(μl) | 1:5(μl) | Vector(μl) |
|---|---|---|---|
| V | 0.8 | 0.8 | 1 |
| G | 2.7 | 4 | |
| R | 5.4 | 7.7 | |
| LT4 | 1 | 1 | 1 |
| LB | 1.1 | 1.5 | 1 |
| H2O | 0 | 0 | 7 |
| Total Volume | 11.0 | 15.0 | 10.0 |
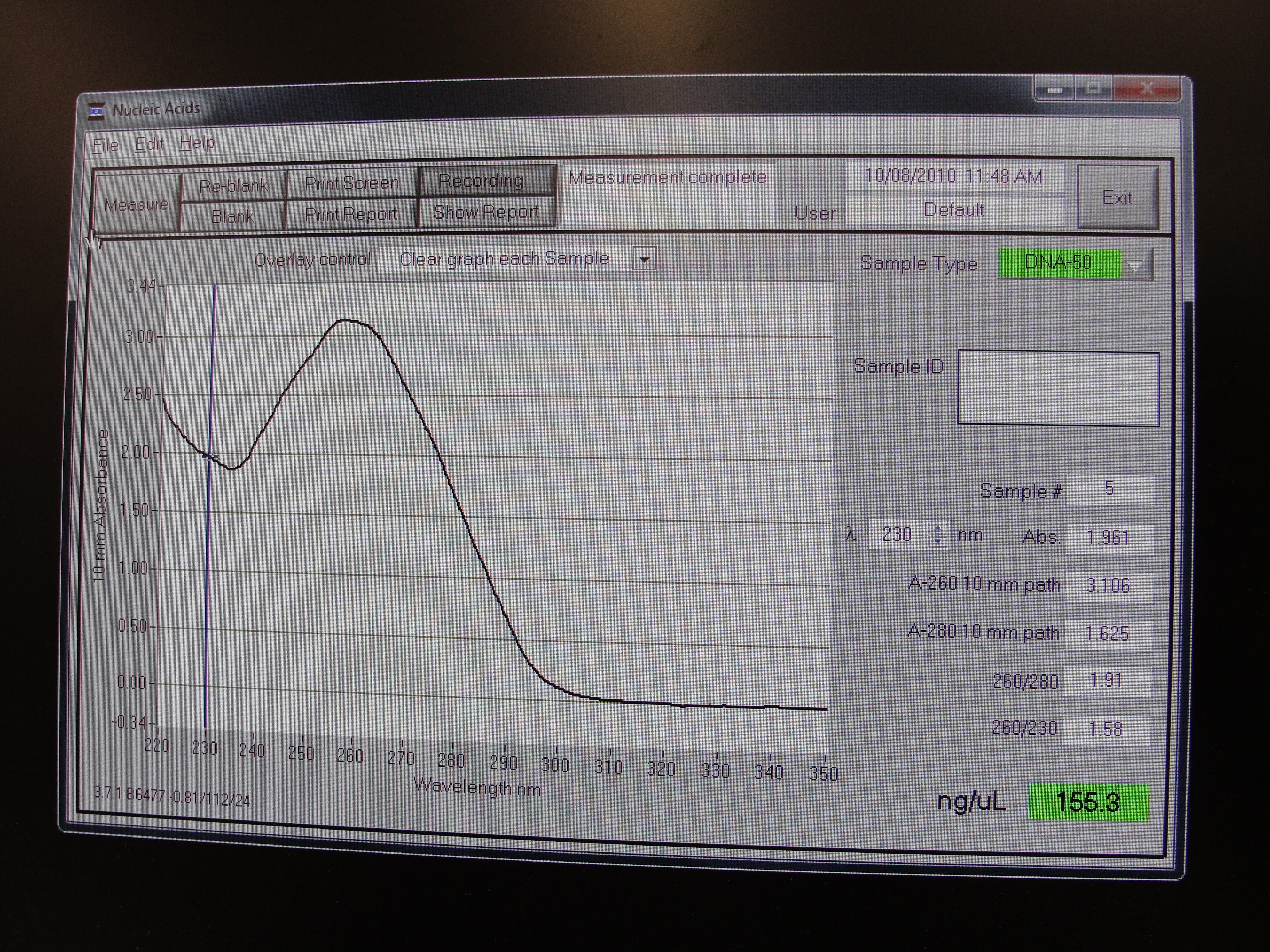
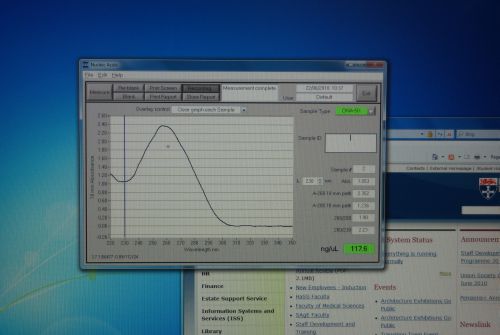
Nanodrop
 
|
 "
"