|
SEPTEMBER: WEEK 4
September, 20th
Screening PCR on 6 colonies picked from I55 was performed. Results were the following:
I55-3 seems to be ok.
Inoculum of I52, I53, I55, I56 and I57 in LB+Amp for MiniPrep of the following day
September, 21st
Miniprep and Nanodrop quantification for:
- I52 : 92,8 ng/ul
- I53 : 118,5 ng/ul
- I55 : 121,1 ng/ul
- I56 : 159,6 ng/ul
- I57 : 103 ng/ul
For I61 the DNA is already available quantified 147,1 ng/ul.
After 3 hour digestion was performed:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 (ul) | Enzyme 2 (ul) | Buffer H (ul)
|
| I52 | Vector | 25 | 10,8 | 9,7 | 1 EcoRI | 1 XbaI | 2,5
|
| I53 | Vector | 25 | 8,5 | 12 | 1 EcoRI | 1 XbaI | 2,5
|
| I55 | Insert/Screening | 25 | 14 | 6,5 | 1 EcoRI | 1 SpeI | 2,5
|
| I56 | Insert/screening | 25 | 10 | 10,5 | 1 XbaI | 1 PstI | 2,5
|
| I57 | Vector | 25 | 9,7 | 10,8 | 1 EcoRI | 1 XbaI | 2,5
|
| I61 | Vector | 25 | 6,8 | 13,7 | 1 EcoRI | 1 XbaI | 2,5
|
Gel run/cut of samples
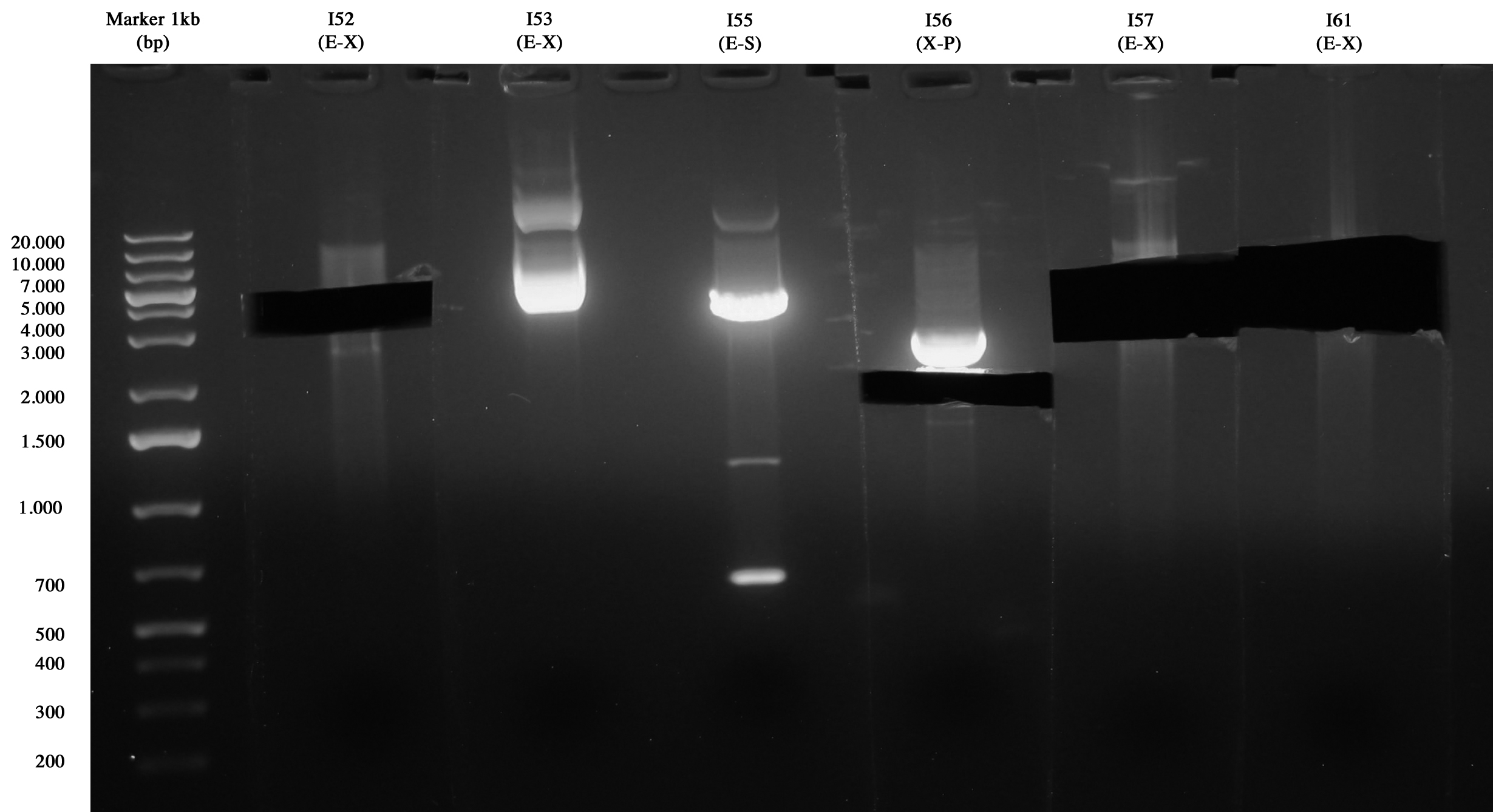 I52(E-X), I53(E-X), I55(E-S), I56(X-P), I57(E-X), I61(E-X). As you can see only I52, I56, I57 and I61 run correctly (I55 was wrong) so gel extraction was performed on these samples:
- I52 = 29,4 ng/ul
- I56 = 15,4 ng/ul
- I57 = 24,7 ng/ul
- I61 = 30,2 ng/ul
We already had digested DNA so we could perform new ON ligations:
- I62 = I39(S-P) + I59(X-P)
- I63 = I38(E-S) + I61(E-X)
- I64 = I58(E-S) + I52(E-X)
- I65 = I39(S-P) + I56(X-P)
- I66 = I60(E-S) + I57(E-X)
Tecan Test on self-inducible promoters was performed on prepared samples with a different protocol that is to bring the cultures to a known OD.
September, 22nd
Trasformation of ligations I62, I63, I64, I65 and I66 into E.coli TOP10.
MiniPrep and quantification of:
- MyYeast1 = 177,8 ng/ul
- MyYeast2 = 98,9 ng/ul
- MyYeast3 = 197,2 ng/ul
Screening digestion was performed as follows:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 (ul) | Enzyme 2 (ul) | Buffer Tango (ul)
|
| MyYeast1 | Screening | 25 | 1,2 | 19,3 | 1 EcoRI | 1 NsiI | 2,5
|
| MyYeast2 | Screening | 25 | 2 | 18,5 | 1 EcoRI | 1 NsiI | 2,5
|
| MyYeast3 | Screening | 25 | 1 | 19,5 | 1 EcoRI | 1 NsiI | 2,5
|
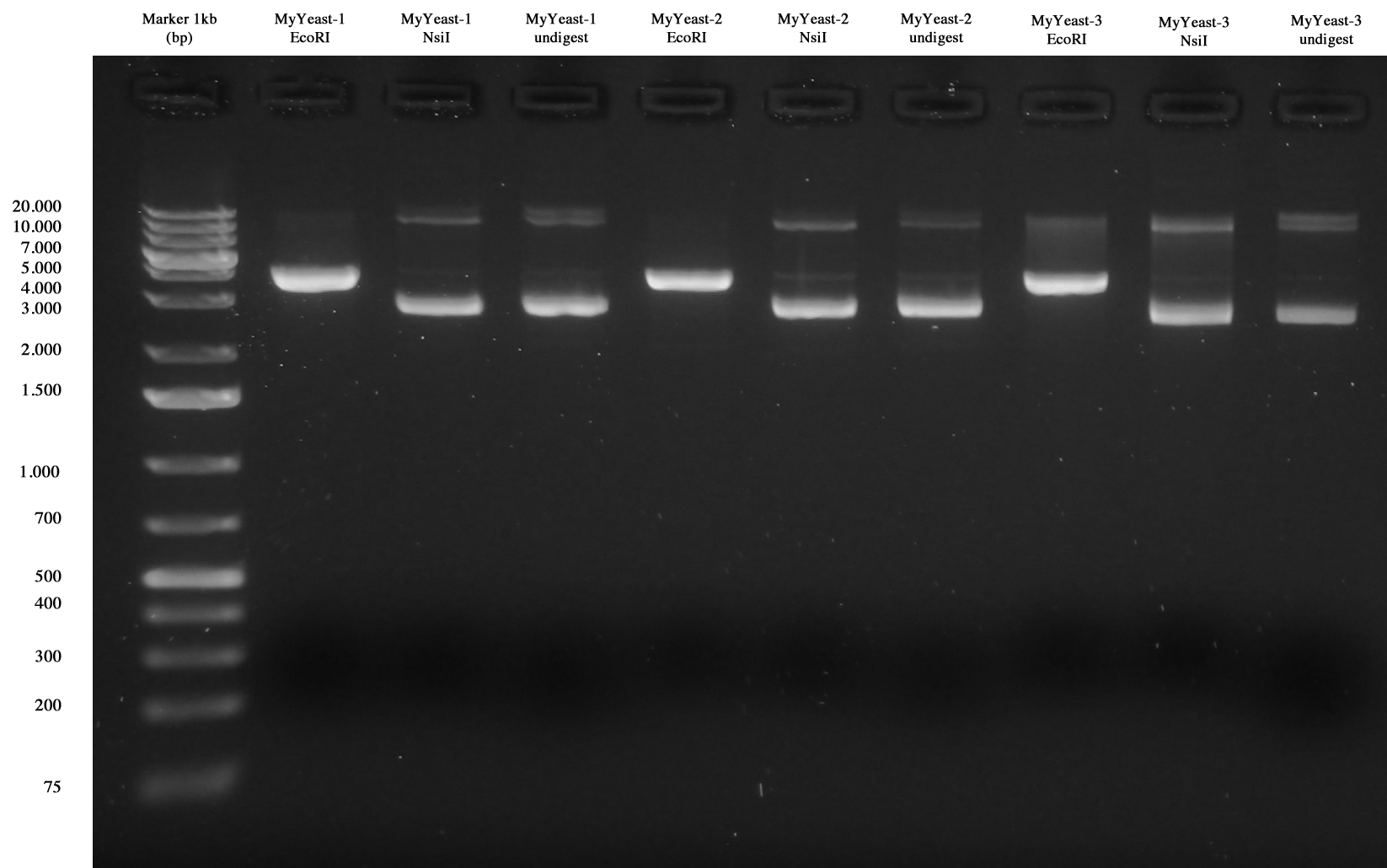
Gel run of samples was performed
 MyYeast-1/2/3 (EcoRI-NsiI digest). None of samples was positive.
Glycerol stocks of MyYeast1, MyYeast2, MyYeast3 was performed.
Inoculum in LB+Amp+Cm12,5 from glycerol stocks and colony for antibiotic resistance testing of I47_S1, I48_S1, I49_S1 and I13522.
September, 23rd
Digestion of yesterday are repeted (because none of them were postitive) as follows:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme (ul) | Buffer H Roche 10X (ul)
|
| MyYeast1 | Screening | 25 | 1,2 | 20,3 | 1 EcoRI | 2,5
|
| MyYeast2 | Screening | 25 | 2 | 19,5 | 1 EcoRI | 2,5
|
| MyYeast3 | Screening | 25 | 1 | 20,5 | 1 EcoRI | 2,5
|
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme (ul) | Buffer Red (ul)
|
| MyYeast1 | Screening | 25 | 1,2 | 20,3 | 1 NsiI | 2,5
|
| MyYeast2 | Screening | 25 | 2 | 19,5 | 1 NsiI | 2,5
|
| MyYeast3 | Screening | 25 | 1 | 20,5 | 1 NsiI | 2,5
|
Gel run of samples was performed
All samples seem to be positive!!
Inoculum for three colonies from I62, I63, I64, I65 agar plates into 5ml LB+Amp.
September, 24th
Glycerol stocks was performed for
- I62-1,2,3
- I63-1,2,3
- I64-1,2,3
- I65-1,2,3
- I66-1,2,3
MiniPrep and Nanodrop quantification :
- I62-1 : 35,1 ng/ul
- I62-2 : 27,8 ng/ul
- I62-3 : 63,8 ng/ul
- I63-1 : 195,3 ng/ul
- I63-2 : 68,3 ng/ul
- I63-3 : 148,7 ng/ul
- I64-1 : 97,8 ng/ul
- I64-2 : 171,8 ng/ul
- I64-3 : 146,3 ng/ul
- I65-1 : 69,7 ng/ul
- I65-2 : 44 ng/ul
- I65-3 : 294,3 ng/ul
- I66-1 : 292,8 ng/ul
- I66-2 : 198 ng/ul
- I66-3 : 284,8 ng/ul
Digestion for screening :
- I62-1,2,3 (E-P)
- I63-1,2,3 (E-P)
- I64-1,2,3 (E-P)
- I65-1,2,3 (E-P)
- I66-1,2,3 (E-P)
Gel run of samples was performed
 Screening for I62/3/4/5/6 (E-P) digested. As you can see I62-1/2/3 were all negative; I63-1/2/3, I64-1/2/3, I65-3, I66-1/2/3 were positive. So we made glycerol stocks for I63-1, I64-2, I65-3, I66-1 and stored them at -80°C. We stored their DNA in -20°C, too.
Dilution 1:50 of I47, I48, I49, GFP in LB+Amp+Cm12,5 and S1 in LB+Cm12,5.
All cultures were grow up to OD=0,1 of Nanodrop. They were then induced with HSL (lysis induction) 100nM
September, 26th
Inoculum of <partinfo>BBa_K173000</partinfo>, I47, I48, I49, <partinfo>BBa_B0031</partinfo> into 3 ml LB+Amp for tomorrow TECAN test. Pick of six colonies from I66 agar plate (I66-A/B/C/D/E/F) and inoculum into 3 ml LB+Amp for screening thorough E-P digest. All colonies were let grow ON at 37°C, 220 rpm.
|
|
 "
"