Team:Brown/Project/Light pattern/Overview
From 2010.igem.org
(Difference between revisions)
(→Overview) |
(→Overview) |
||
| Line 18: | Line 18: | ||
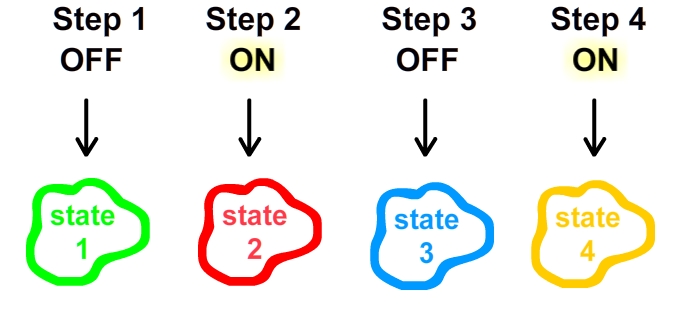
[[Image:theNew.jpg|thumb|300px|right|As the light alternates from OFF/ON, our circuit progresses to a third and fourth state!]] | [[Image:theNew.jpg|thumb|300px|right|As the light alternates from OFF/ON, our circuit progresses to a third and fourth state!]] | ||
| - | What are the ramifications of this project? We believe that our circuit design, where progression across sets of protein production is controlled solely by light, has the potential to drastically reduce costs in biological manufacturing practices. Furthermore, we hope our project provides a foundational advancement in circuit design by emphasizing the importance of '''abstraction'''; specifically, the separation of user input and the precise molecules that control transcription. Read on to learn more about our project! | + | What are the ramifications of this project? We believe that our circuit design, where progression across sets of protein production is controlled solely by light, has the potential to drastically reduce costs in biological manufacturing practices. Furthermore, we hope our project provides a foundational advancement in circuit design by emphasizing the importance of '''abstraction'''; specifically, the separation of user input and the precise molecules that control transcription. [[Team:Brown/project/Light_pattern/Logic_design| Read on]] to learn more about our project! |
===='''References'''==== | ===='''References'''==== | ||
1) Valdez-Cruz et al. Microbial Cell Factories. 2010, 9:18 | 1) Valdez-Cruz et al. Microbial Cell Factories. 2010, 9:18 | ||
Revision as of 17:06, 27 October 2010
Light-Pattern Controlled Circuit
Overview
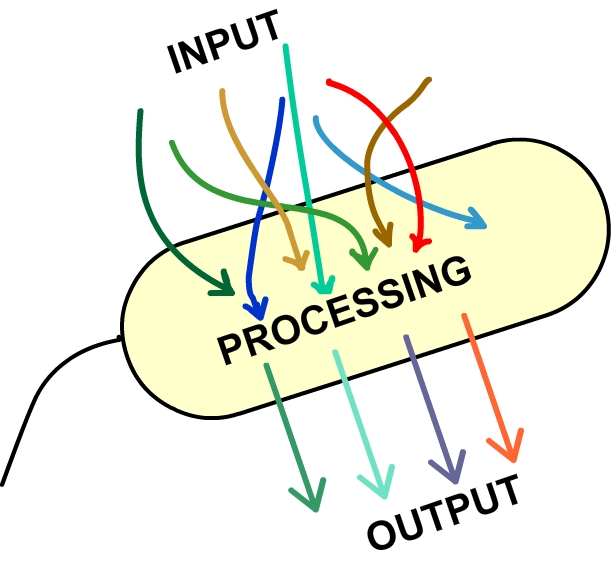
Our project this year attempts to tackle what we see as emerging issue in synthetic biology: the complexity of circuit inputs. There is a developing trend among iGEM projects towards intricate systems; the registry of standard parts is growing quickly and the tools at the disposal of a synthetic biologist are increasing rapidly. While many of these systems rely on an autonomous progression of events in the chasis - say, a cell encounters some environmental stimulus which triggers downstream responses - some require precise user control. This is especially true for projects in the manufacturing area, as often a product is achieved following a progression of steps within the cell. Functioning "behind the scenes" to achieve this sort of controllable progression is the genetic circuit, consisting of plasmids loaded with promoter and transcription factor pairs. Through a combination of regulators and activators, these circuits achieve distinct "states," or set of functions carried out by the cell. Whether chemical, heat, or some other environmental change, it is common practice that for each state, a unique input must be applied. With this approach, it is quite clear that an increasingly complicated circuit, with many different promoters, requires an increasingly intricate set of inputs.We believe this to be a problem for a few reasons:
- Accessibility
- From an end user perspective, navigation across states could become nightmarish. Especially for a system targeted beyond the lab, perhaps at a non-technical setting, input/control must be accessible and easily attainable.
- Expense
- Chemical induction of individual components of a circuit, particularly on a large bioreactor scale as in telescoping synthesis, is expensive!1 For a full-fledged, industrial bioreactor, an enormous amount of inducer must be introduced. As this chemical inducer can be toxic, it must be removed from the end product, adding to the expense of manufacturing.1 One chemical input alone on this scale can vastly increase the cost of the end product; multiple chemical inputs drives costs extremely high.
- Efficiency
- Environmental changes, such as heat or pH, may remove a cell from optimal conditions for protein production. Furthermore, the timing of environmental changes can be difficult and imprecise, distancing the system from its peak production capabilities1. Again, from a manufacturing standpoint, a decrease in yield per reaction increases costs and decreases overall efficiency.
Rather than returning to state 1 given a light change back to OFF, our circuit takes advantage of a memory component to effect a unique progression of states.
What are the ramifications of this project? We believe that our circuit design, where progression across sets of protein production is controlled solely by light, has the potential to drastically reduce costs in biological manufacturing practices. Furthermore, we hope our project provides a foundational advancement in circuit design by emphasizing the importance of abstraction; specifically, the separation of user input and the precise molecules that control transcription. Read on to learn more about our project!
References
1) Valdez-Cruz et al. Microbial Cell Factories. 2010, 9:18
 "
"