Team:SDU-Denmark/labnotes3
From 2010.igem.org
(Difference between revisions)
(→Pfu PCR of J13002 and B0015) |
(→colony PCR using taq on ligated BBa_J13002 in pSB1C3) |
||
| (19 intermediate revisions not shown) | |||
| Line 124: | Line 124: | ||
The Concentrations were quite low, but we pooled them (1P+2P and 3B+4B) and used them for ligation. | The Concentrations were quite low, but we pooled them (1P+2P and 3B+4B) and used them for ligation. | ||
<br><br> | <br><br> | ||
| + | |||
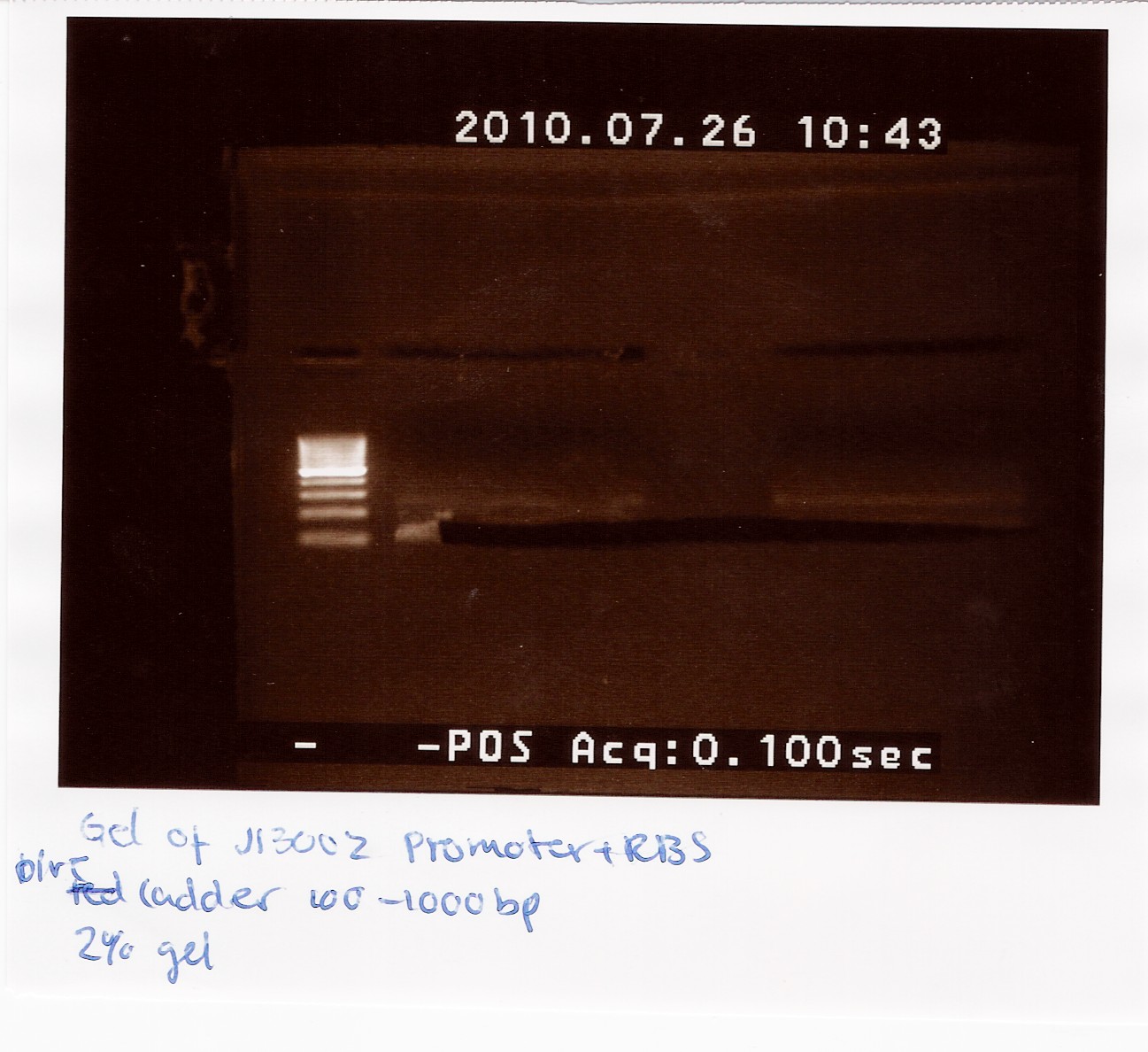
| + | ==== Ligation of J13002 promotor rbs into pSB3T5 assembly plasmid. ==== | ||
| + | Start date: 26/7<br> | ||
| + | Methods: Restriction Digest, Gel Extraction, Ligation, Transformation (not done by me) and colony pcr<br> | ||
| + | Protocols: CP1.3, LG1.3, RD1.1, DE1.3 | ||
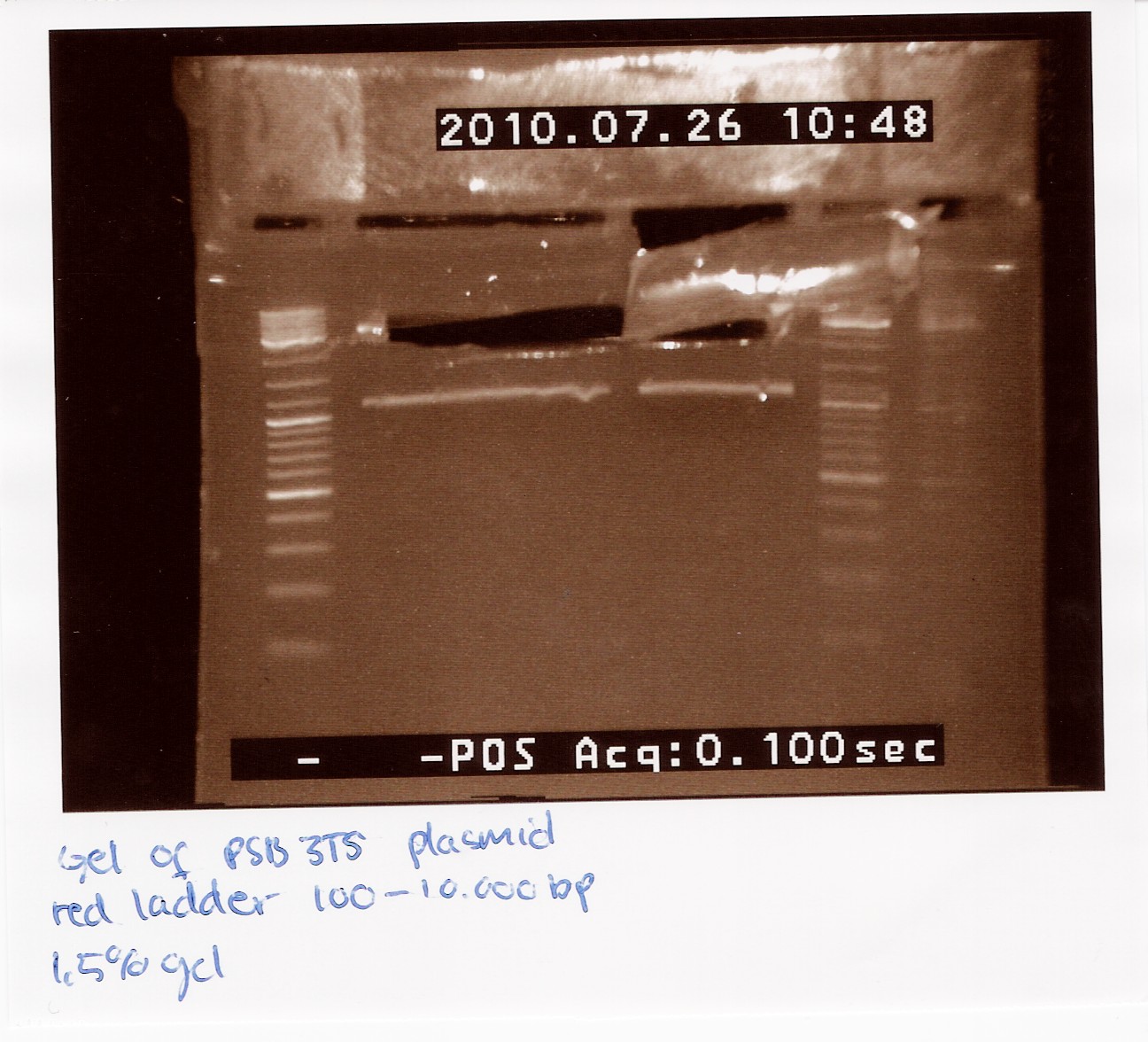
| + | ==== Restriction digest, Gel extracton and ligation of BBa_J13002 and pSB1C3 ==== | ||
| + | Date: 27/7<br> | ||
| + | Done by: Christian<br> | ||
| + | Methods: Restriction digest, Gel extraction, ligation and transformation (not by me)<br> | ||
| + | protocos: RD1.1, DE1.3, LG1.3 | ||
| + | <br><br> | ||
| + | Restriction digest using EcoRI and PstI and gel extraction were done following protocol. at beginning of ligation concentrations were: 3.7ng/ul part at 95BP and 8ng/ul plasmid at 3215BP. four tubes were run, 1 at 3:1 ration, two at 6:1 and one at 9:1. iGEM consensus protocol was followed, with 20ng vector as starting point. Tubes were cooled for transformations in the morning. | ||
| + | <br><br> | ||
| + | Results: Quite low concentrations were reached, but the iGEM protocol should ensure the same molar ratio and total concentration as was planned for.<br><br> | ||
| + | Analysis: Everything is going according to plan for now. | ||
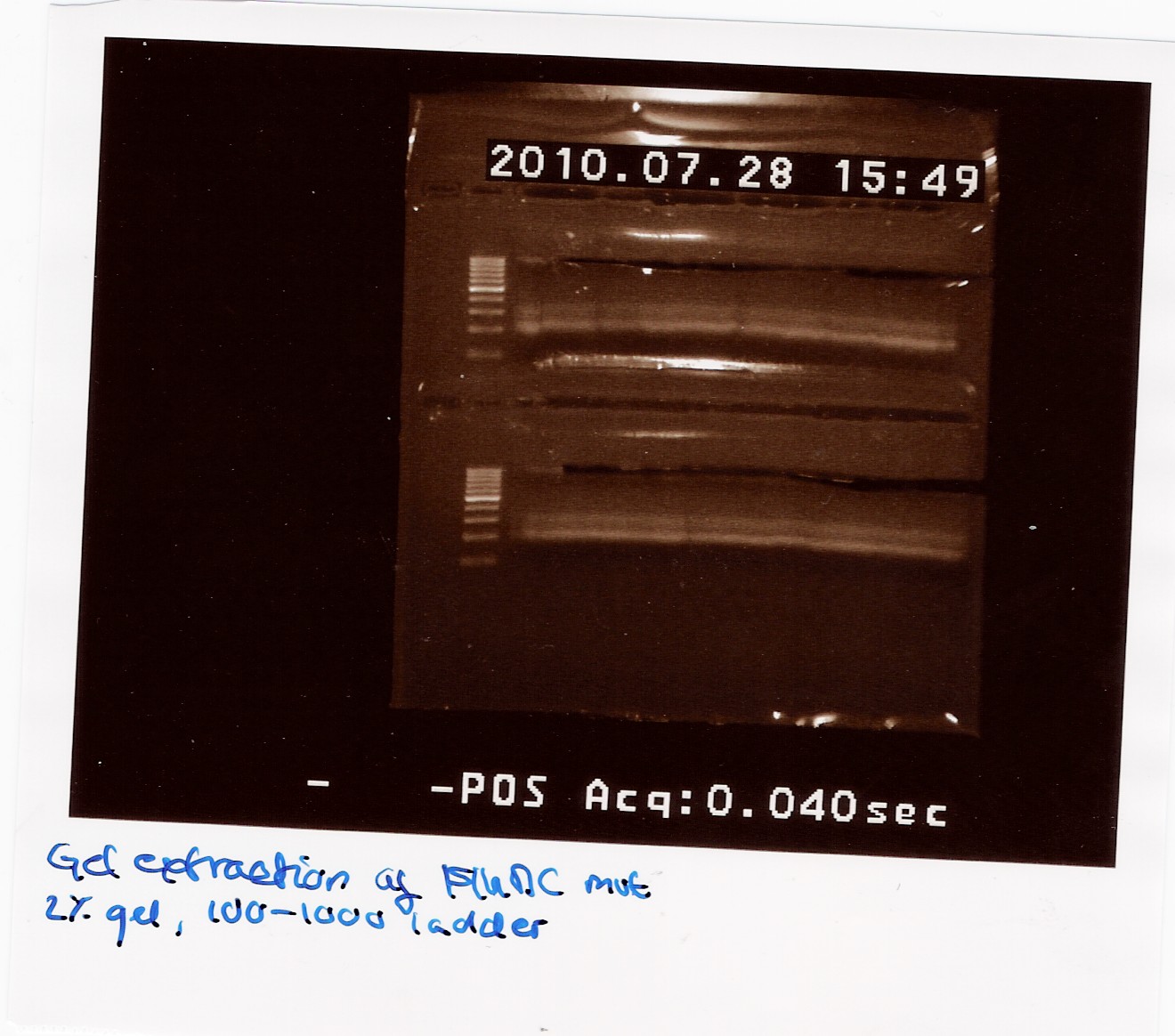
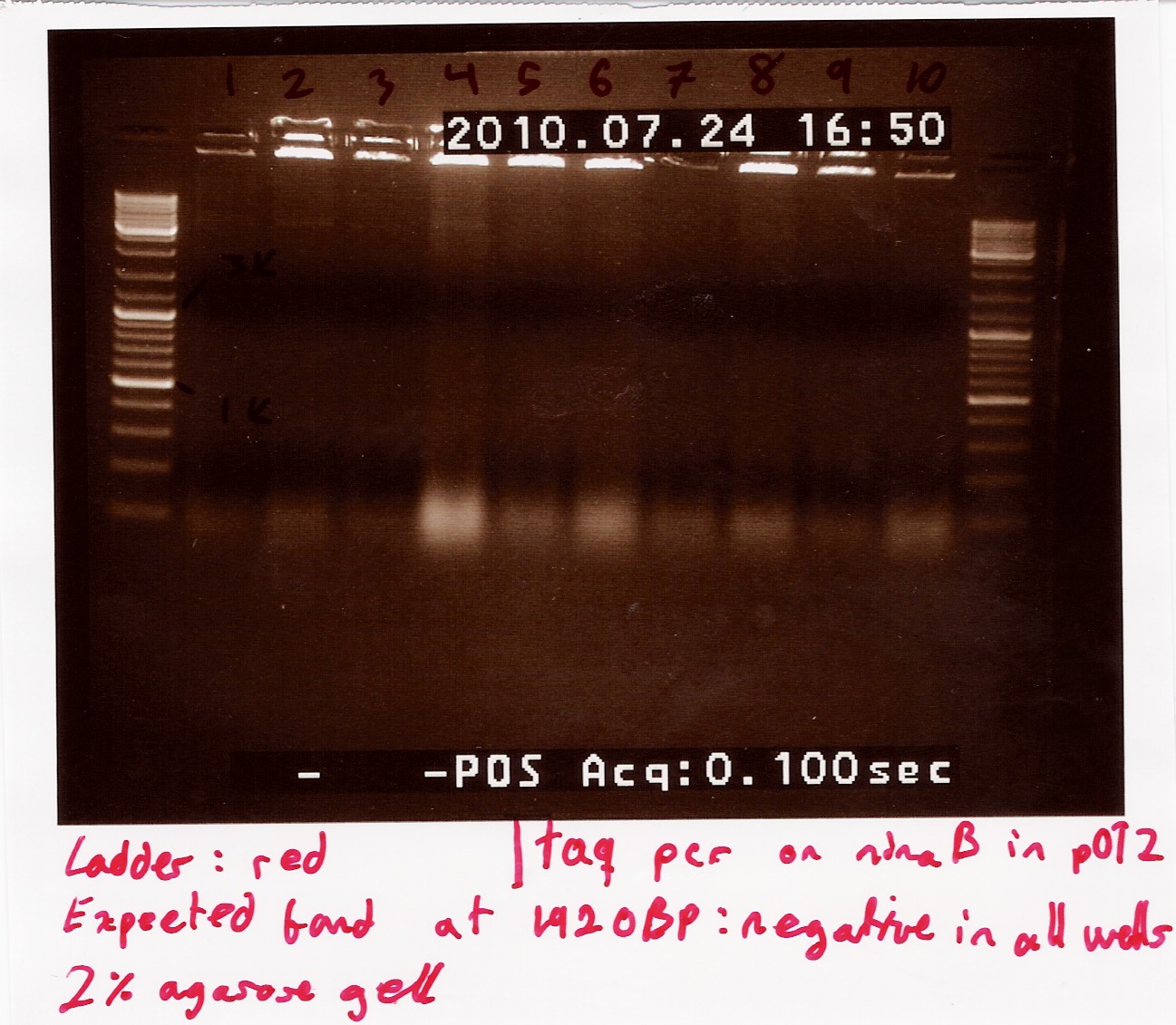
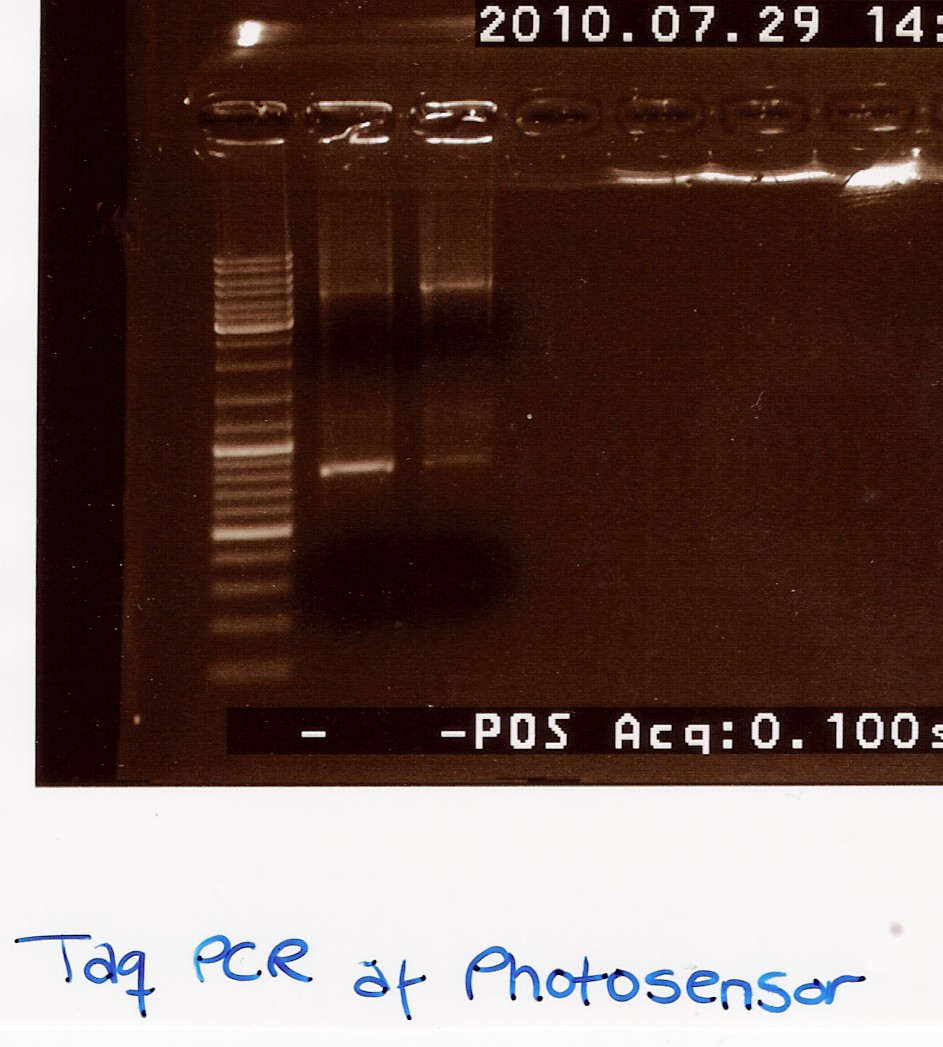
| + | ==== colony PCR using taq on ligated BBa_J13002 in pSB1C3 ==== | ||
| + | Date: 28/7<br> | ||
| + | Done by: Christian<br> | ||
| + | Methods: colony pcr<br> | ||
| + | protocos: CP1.3 | ||
| + | <br><br> | ||
| + | Colony pcr was run on 8 colonies, and plates were streaked and incubated at 37° ON. protocol was followed to point, with enzyme added to mix. PErhaps too little colony was scraped of original plates, and some tubes were defective, letting out steam during pcr. | ||
| + | <br><br> | ||
| + | Results: A band showed at around 300bp in well 4.1. The expected length was 312bp. wells3.3 and 3.4 show a band outside the blue ladder, that could be rfp (since this was the original insert.) tube 3.2 was ruined.<br> | ||
| + | [[Image:Team-sdu-denmark-Promotor_Rbs_i_psb3t5_colonipcr.jpg | 300px]] | ||
| + | <br><br> | ||
| + | Analysis: Well 3.2 shows promising results. ON and miniprep will be made, and it will be sent for sequencing.<br><br> | ||
=== Pfu PCR of J13002 and B0015 === | === Pfu PCR of J13002 and B0015 === | ||
| Line 275: | Line 302: | ||
=== Pfu PCR and gel purification of pSB1Ak3 w. B0015 === | === Pfu PCR and gel purification of pSB1Ak3 w. B0015 === | ||
| - | |||
''Done by:'' Pernille <br> | ''Done by:'' Pernille <br> | ||
''Date:'' July 28th <br> | ''Date:'' July 28th <br> | ||
| - | ''Protocol:'' [[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP1.1]], [[https://2010.igem.org/Team:SDU-Denmark/protocols#DE1.2 Gel | + | ''Protocol:'' [[https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP1.1]], [[https://2010.igem.org/Team:SDU-Denmark/protocols#DE1.2 Gel extraction]]<br> |
''Notes:'' <br> the PCR was run with the following program: | ''Notes:'' <br> the PCR was run with the following program: | ||
<html> | <html> | ||
| Line 605: | Line 631: | ||
--[[User:Louch07|Louch07]] 09:06, 29 July 2010 (UTC) | --[[User:Louch07|Louch07]] 09:06, 29 July 2010 (UTC) | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
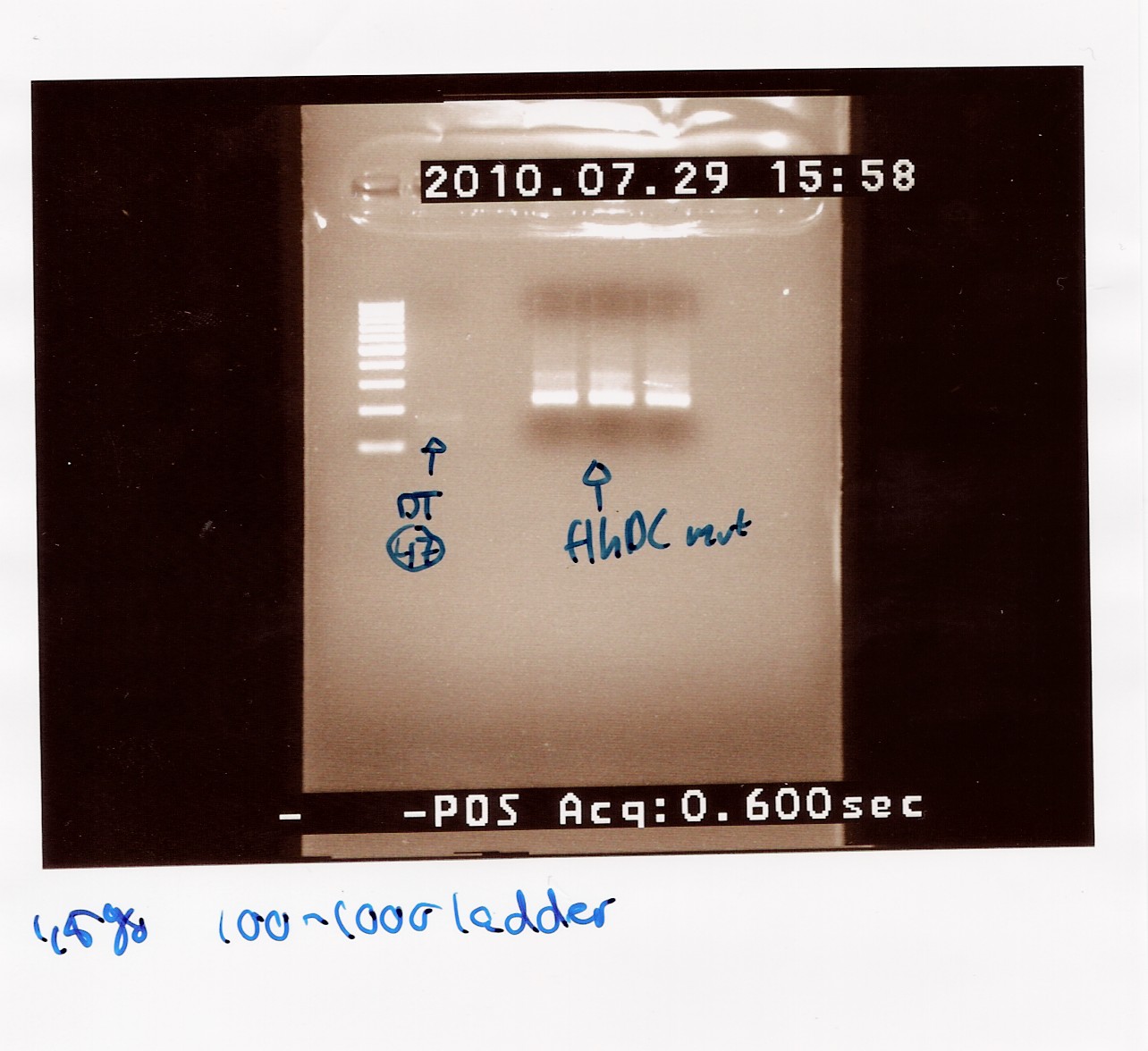
=== PCR of FlhDC freeze tube 23 === | === PCR of FlhDC freeze tube 23 === | ||
<br> | <br> | ||
| Line 684: | Line 671: | ||
''Notes:'' purified flhD/C and miniprep of pSB1C3 and pSB3K3 were digested using the EcoRI and PstI restriction enzymes. <br><br> | ''Notes:'' purified flhD/C and miniprep of pSB1C3 and pSB3K3 were digested using the EcoRI and PstI restriction enzymes. <br><br> | ||
Restriction mixture (FlhD/C):<br> | Restriction mixture (FlhD/C):<br> | ||
| - | <html><table style="text-align: left; width: | + | <html><table style="text-align: left; width: 300px;" border="1" |
cellpadding="2" cellspacing="2"> | cellpadding="2" cellspacing="2"> | ||
<tr> | <tr> | ||
| Line 708: | Line 695: | ||
</table><br> | </table><br> | ||
Restriction mixture (plasmid):<br> | Restriction mixture (plasmid):<br> | ||
| - | <table style="text-align: left; width: | + | <table style="text-align: left; width: 300px;" border="1" |
cellpadding="2" cellspacing="2"> | cellpadding="2" cellspacing="2"> | ||
<tr> | <tr> | ||
| Line 737: | Line 724: | ||
DNA was extracted from gel according to protocol<br><br> | DNA was extracted from gel according to protocol<br><br> | ||
''Results:''<br><br> | ''Results:''<br><br> | ||
| + | |||
==== Ligation ==== | ==== Ligation ==== | ||
''Protocol:'' [https://2010.igem.org/Team:SDU-Denmark/protocols#LG1.2 LG1.2]<br><br> | ''Protocol:'' [https://2010.igem.org/Team:SDU-Denmark/protocols#LG1.2 LG1.2]<br><br> | ||
| Line 826: | Line 814: | ||
=== Follow-up colony PCR === | === Follow-up colony PCR === | ||
| - | Date: 26/7<br> | + | ''Date:'' 26/7<br> |
| - | Methods: Colony PCR<br> | + | ''Methods:'' Colony PCR<br> |
| - | Protocol: CP1.3<br> | + | ''Protocol:'' CP1.3<br> |
Experiment done by: Maria, LC | Experiment done by: Maria, LC | ||
<br><br> | <br><br> | ||
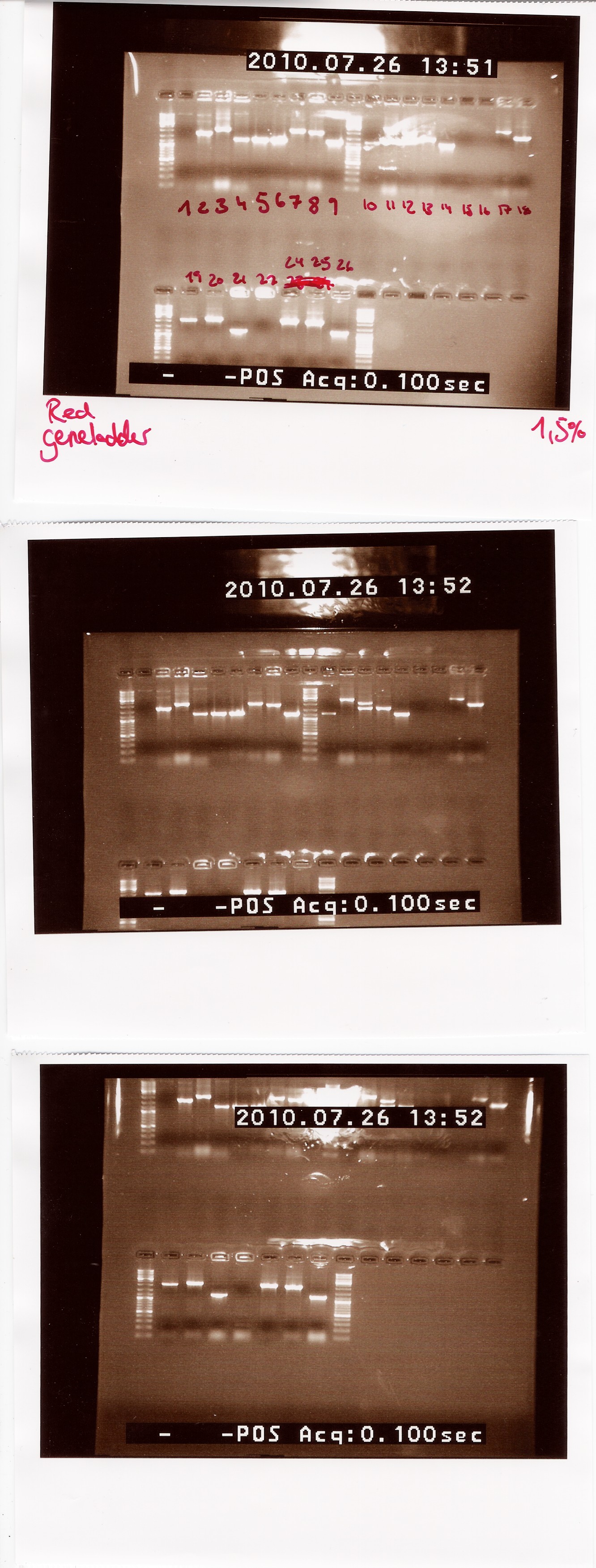
| - | Notes: We made a sample for every plate from the last colony PCR. Out of the 29 samples we could only run 25 at once in the PCR machine. Plate 23 was missing, so there is no sample for it.<br><br> | + | ''Notes:'' We made a sample for every plate from the last colony PCR. Out of the 29 samples we could only run 25 at once in the PCR machine. Plate 23 was missing, so there is no sample for it.<br><br> |
| - | Results: [[Image:Team-SDU-Denmark-colpcr267.jpg| | + | ''Results:'' <br> |
| + | [[Image:Team-SDU-Denmark-colpcr267.jpg|300px]]<br> | ||
Lengths of PCR products: <br> | Lengths of PCR products: <br> | ||
1200: 4, 5, 6, 9, 10, 14, 21, 26<br> | 1200: 4, 5, 6, 9, 10, 14, 21, 26<br> | ||
| Line 843: | Line 832: | ||
Colonies 1, 15, 16 and 22 gave no result. | Colonies 1, 15, 16 and 22 gave no result. | ||
<br><br> | <br><br> | ||
| - | Analysis: Since we had so many different lengths, we will cut one from each length, specifically colony: 2, 8, 11, 13, 20, 24, 26. | + | ''Analysis:'' Since we had so many different lengths, we will cut one from each length, specifically colony: 2, 8, 11, 13, 20, 24, 26. |
<br><br> | <br><br> | ||
| Line 1,165: | Line 1,154: | ||
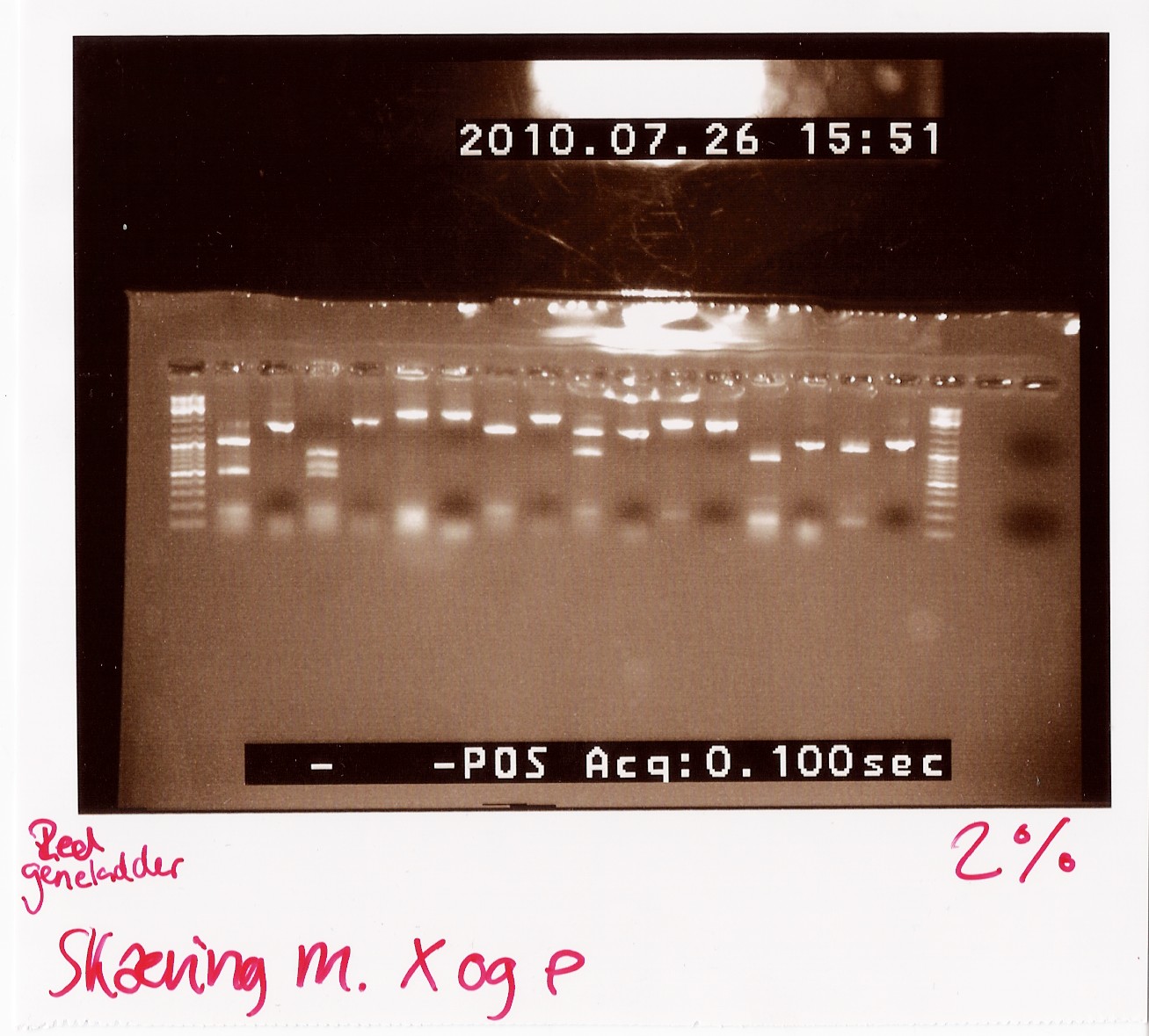
''Results:''<br> | ''Results:''<br> | ||
gel electrophoresis:<br> | gel electrophoresis:<br> | ||
| - | [[Image:Team-SDU-Denmark-Digestwithxandp.jpg| | + | [[Image:Team-SDU-Denmark-Digestwithxandp.jpg|300px]]<br><br> |
''Analysis:''<br> | ''Analysis:''<br> | ||
The lanes containing digested PCR product from coloni #5, 6 and 21 have a band smear around 100-200bp,and a band at around 800bp.<br> | The lanes containing digested PCR product from coloni #5, 6 and 21 have a band smear around 100-200bp,and a band at around 800bp.<br> | ||
| Line 1,212: | Line 1,201: | ||
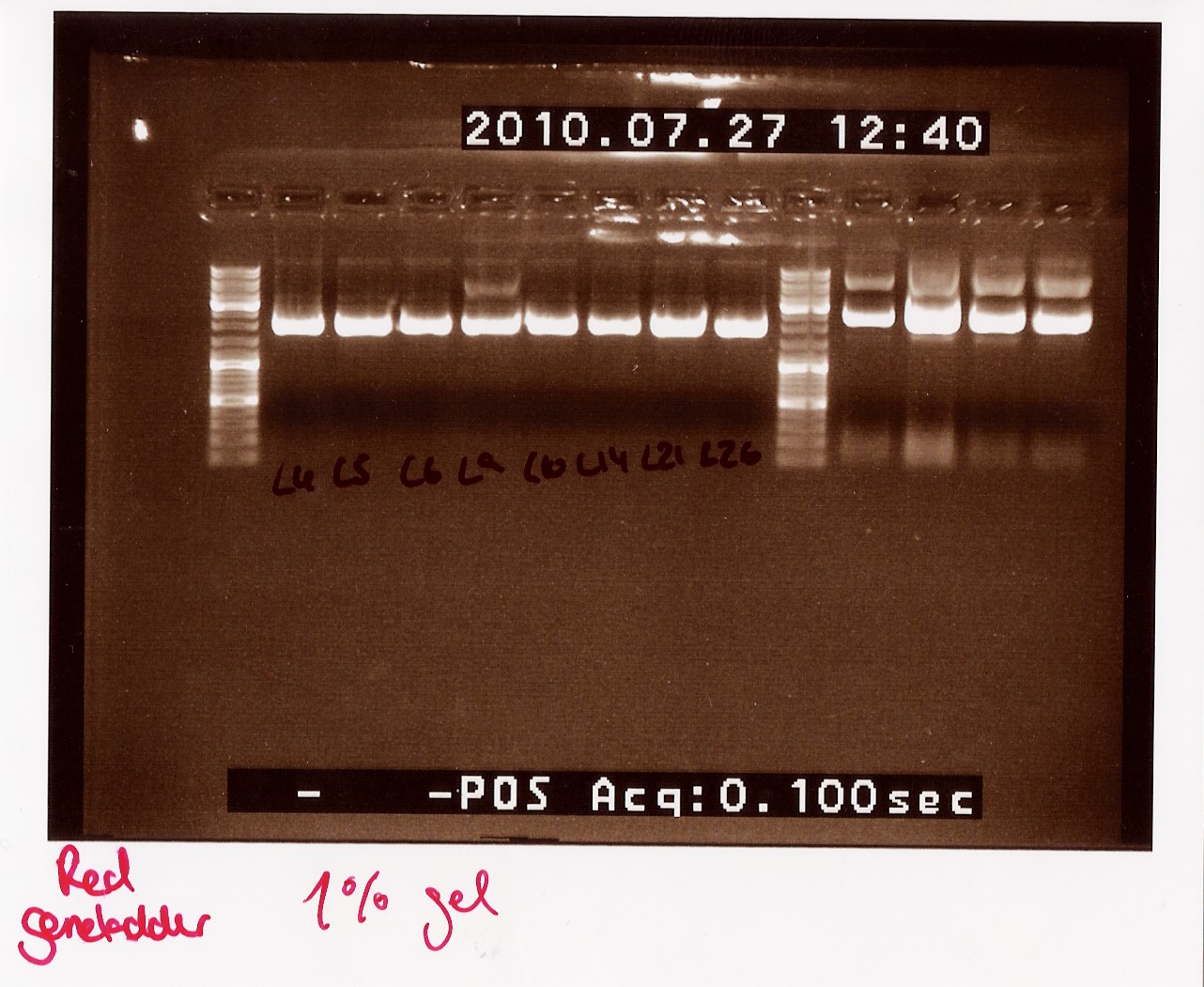
''Results:''<br> | ''Results:''<br> | ||
Gel electrophoresis:<br> | Gel electrophoresis:<br> | ||
| - | [[Image:Team-SDU-Denmark-Miniprepcoloni5,6,21.jpg| | + | [[Image:Team-SDU-Denmark-Miniprepcoloni5,6,21.jpg|300px]]<br><br> |
''Analysis:''<br> | ''Analysis:''<br> | ||
we have purified the plasmids.The concentrations are high but not as high as expected when the cells are boosted prior to the miniprep.<br> In order to obtain an even higher concentration transfer all 5mL ON culture to 20mL pre heated LB media. run all 25mL new culture as 1 miniprep.<br> Due to the low concentrations we need to dry down our samples before they are ready for sequentation.<br> | we have purified the plasmids.The concentrations are high but not as high as expected when the cells are boosted prior to the miniprep.<br> In order to obtain an even higher concentration transfer all 5mL ON culture to 20mL pre heated LB media. run all 25mL new culture as 1 miniprep.<br> Due to the low concentrations we need to dry down our samples before they are ready for sequentation.<br> | ||
| Line 1,238: | Line 1,227: | ||
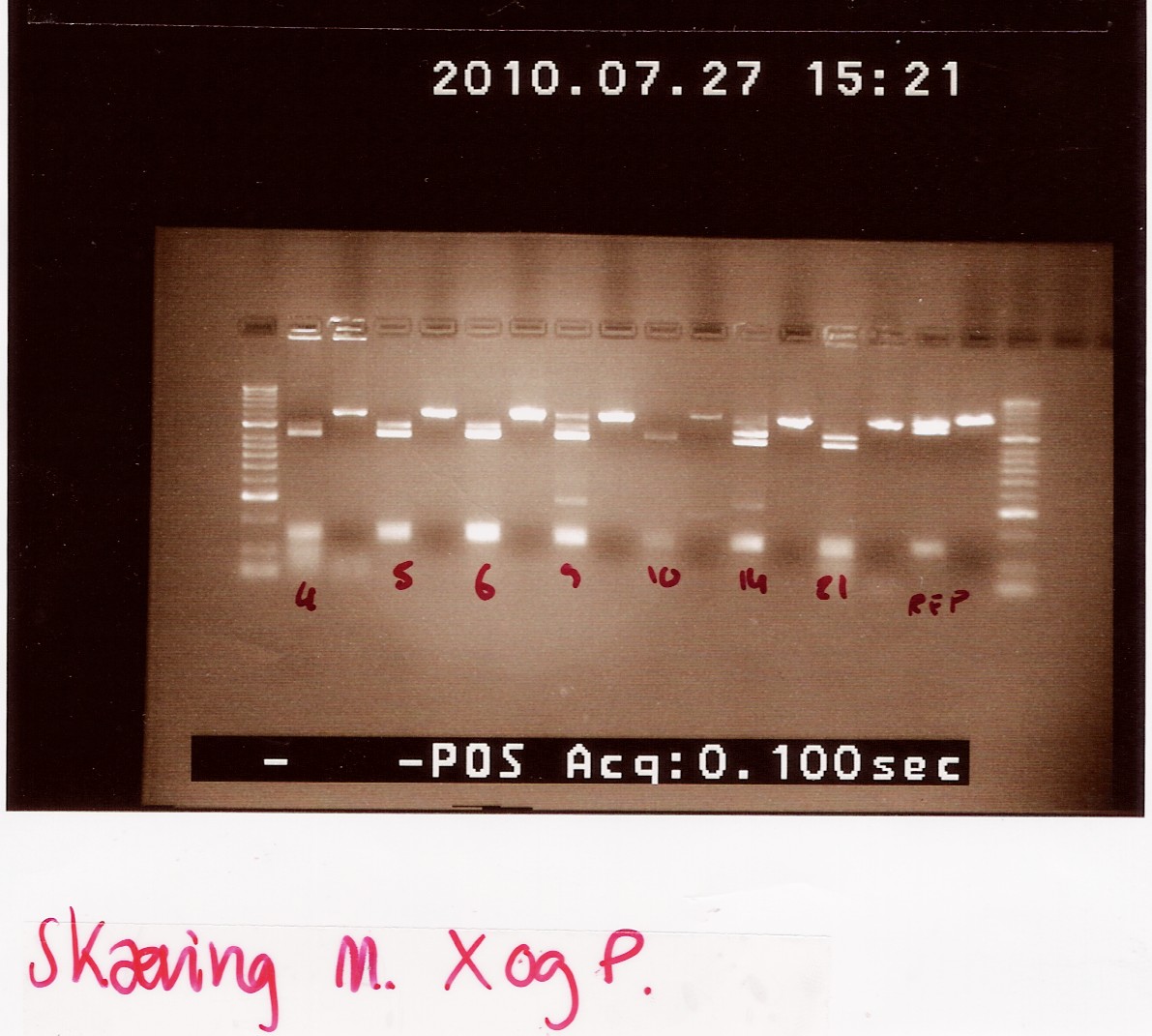
<br><br> | <br><br> | ||
Results:_Expected length at 1920 BP did not show. In fact no bands showed, except somethin like a primer smear around 100bp.<br> | Results:_Expected length at 1920 BP did not show. In fact no bands showed, except somethin like a primer smear around 100bp.<br> | ||
| - | [[Image:Team-SDU-Denmark-NinaB taq colony pcr on transformants.jpg | | + | [[Image:Team-SDU-Denmark-NinaB taq colony pcr on transformants.jpg | 300px ]] |
<br><br> | <br><br> | ||
Analysis: Anealing temperature might have been set to high, as the primers were constructed to aneal at 55°. another run will be done on miniprepped plasmids, with a different program. | Analysis: Anealing temperature might have been set to high, as the primers were constructed to aneal at 55°. another run will be done on miniprepped plasmids, with a different program. | ||
| + | |||
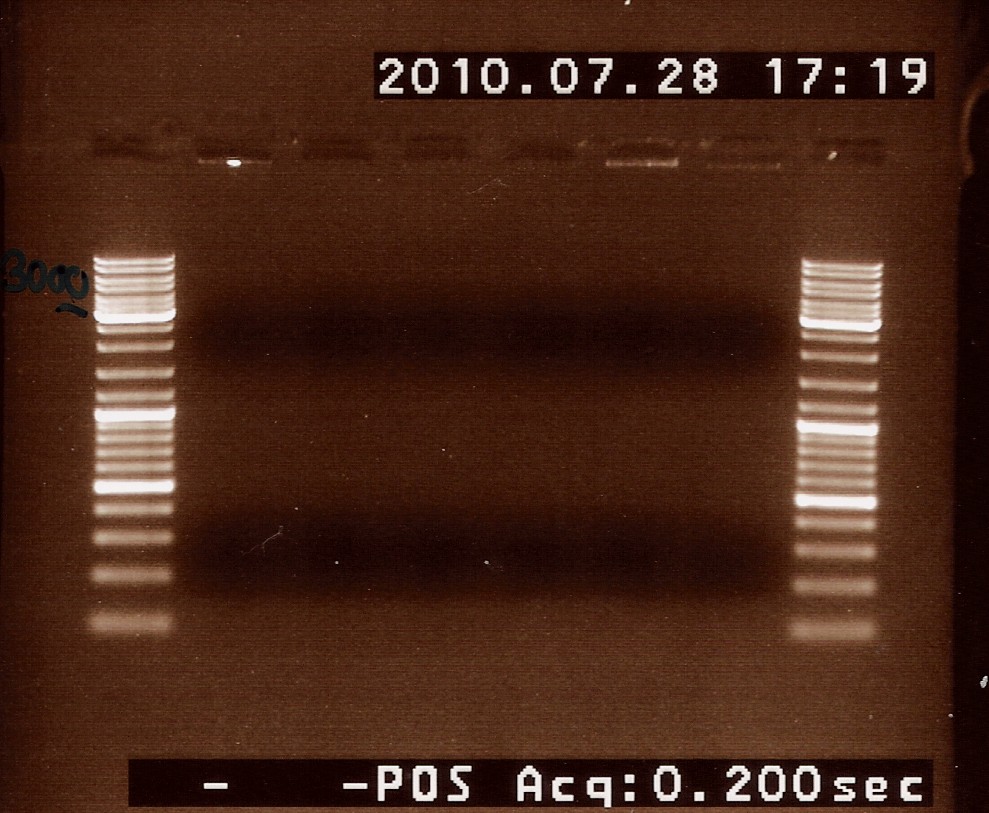
==== Restriction digest on miniprepped pOT2 plasmids with ninaB insert using EcoRI ==== | ==== Restriction digest on miniprepped pOT2 plasmids with ninaB insert using EcoRI ==== | ||
Date: 27/7<br> | Date: 27/7<br> | ||
| Line 1,250: | Line 1,240: | ||
<br><br> | <br><br> | ||
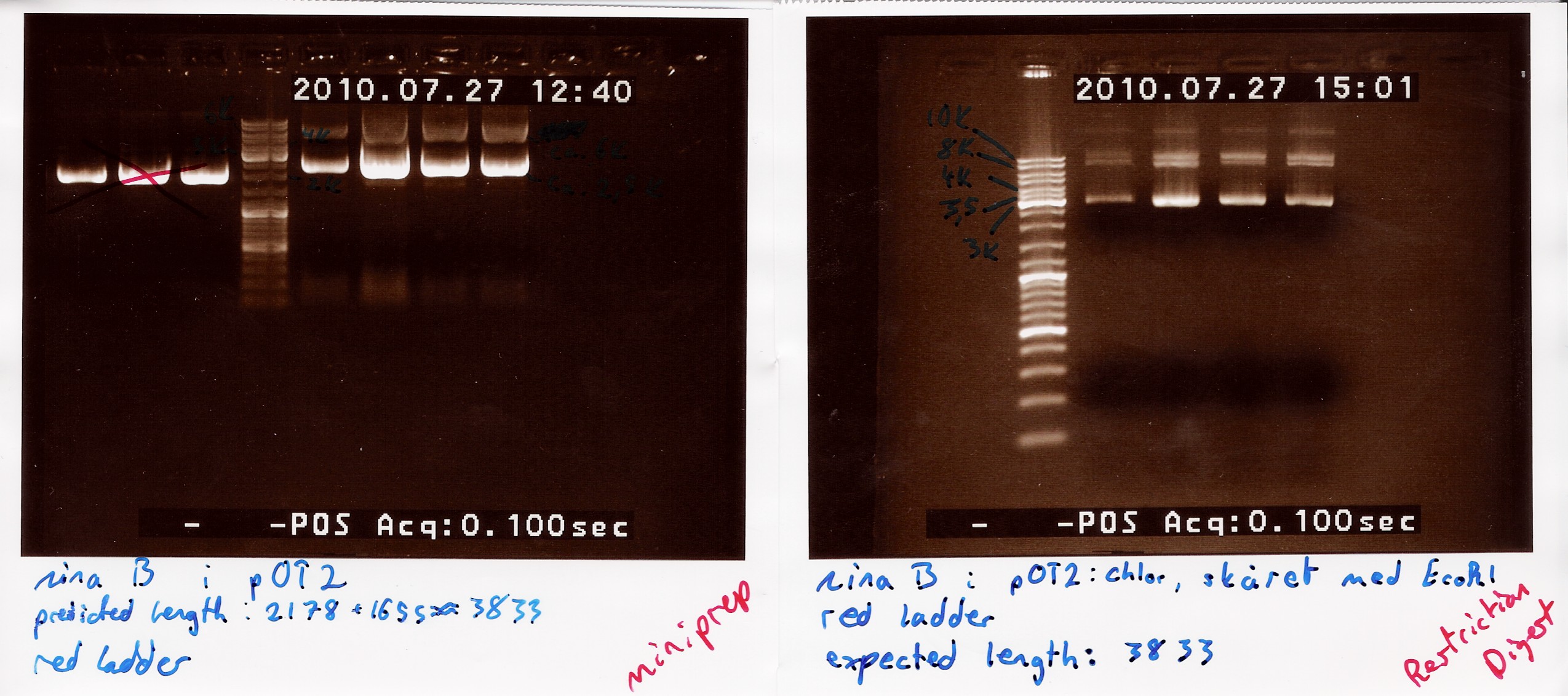
Results: strange things going on at different lengths, and nothing matches the expected length of about 3800BP.<br> | Results: strange things going on at different lengths, and nothing matches the expected length of about 3800BP.<br> | ||
| - | [[Image:Team-SDU-Denmark-NinaB miniprep and first restriction digest-1.jpg | | + | [[Image:Team-SDU-Denmark-NinaB miniprep and first restriction digest-1.jpg |500px]] |
<br> | <br> | ||
Analysis: Another digest is needed to show that plasmid length is wrong, as it seems unlikely with comercial plasmids.<br> | Analysis: Another digest is needed to show that plasmid length is wrong, as it seems unlikely with comercial plasmids.<br> | ||
| + | |||
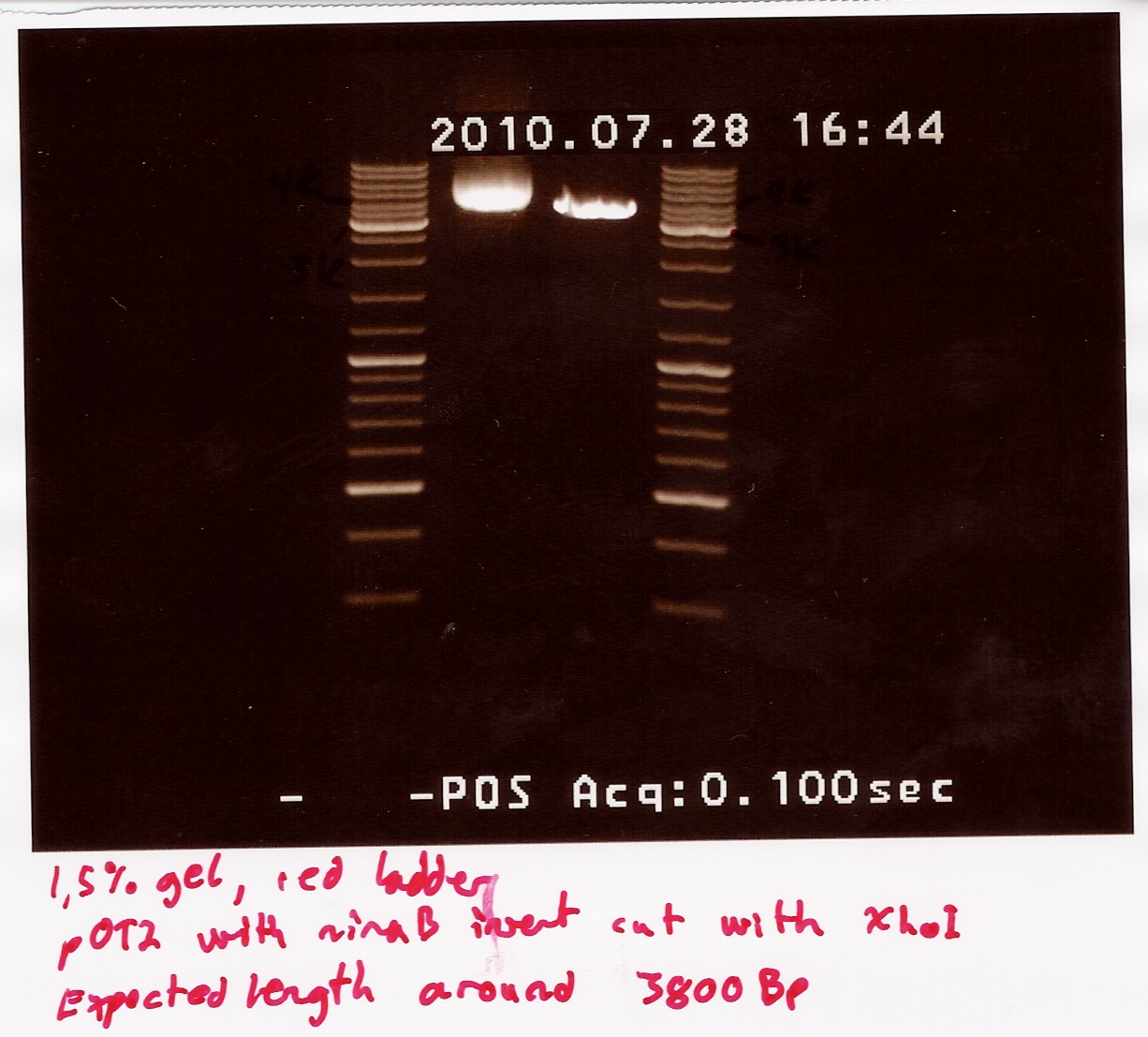
==== Restriction digest on miniprepped pOT2 plasmids with ninaB insert using EcoRI and XhoI ==== | ==== Restriction digest on miniprepped pOT2 plasmids with ninaB insert using EcoRI and XhoI ==== | ||
Date: 28/7<br> | Date: 28/7<br> | ||
| Line 1,262: | Line 1,253: | ||
<br><br> | <br><br> | ||
results: Digest showed a band close to the 3.8kb marker expected for the full length plasmid, concurrent with only one digest working. (although an earlier digest with EcoRI alone showed a different band.) Miniprepped plasmid had also "moved up" the ladder<br> | results: Digest showed a band close to the 3.8kb marker expected for the full length plasmid, concurrent with only one digest working. (although an earlier digest with EcoRI alone showed a different band.) Miniprepped plasmid had also "moved up" the ladder<br> | ||
| - | [[Image:Team-SDU-Denmark-NinaB restriction digest with eco and xho-1.jpg | | + | [[Image:Team-SDU-Denmark-NinaB restriction digest with eco and xho-1.jpg | 300px ]] |
<br><br> | <br><br> | ||
analysis: Something fishy is up with this plasmid, and the digest. one more will be run only using XhoI. | analysis: Something fishy is up with this plasmid, and the digest. one more will be run only using XhoI. | ||
| + | |||
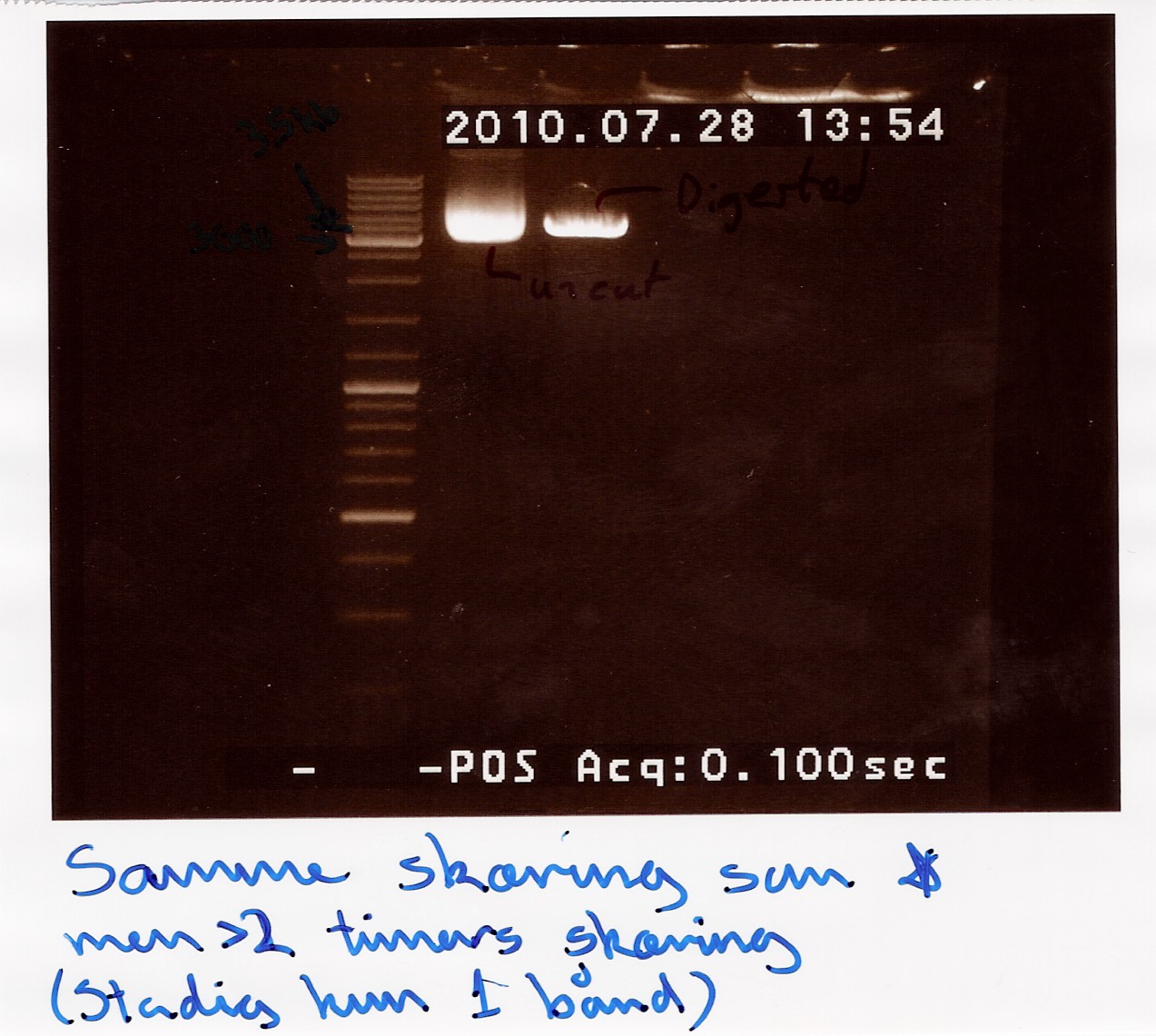
==== Restriction digest on miniprepped pOT2 plasmids with ninaB insert using EcoRI and XhoI ==== | ==== Restriction digest on miniprepped pOT2 plasmids with ninaB insert using EcoRI and XhoI ==== | ||
Date: 28/7<br> | Date: 28/7<br> | ||
| Line 1,274: | Line 1,266: | ||
<br><br> | <br><br> | ||
results: Gel showed a band between 3.5K and 4k markers, consistent with earlier results, indicating that the plasmid and insert have correct length. Strangely the cut segment has traveled further through the gell than the un-cut control.<br> | results: Gel showed a band between 3.5K and 4k markers, consistent with earlier results, indicating that the plasmid and insert have correct length. Strangely the cut segment has traveled further through the gell than the un-cut control.<br> | ||
| - | [[Image:Team-SDU-Denmark-NinaB restriction digest with xhoI.jpg | | + | [[Image:Team-SDU-Denmark-NinaB restriction digest with xhoI.jpg | 300px ]] |
<br><br> | <br><br> | ||
analysis: There realy is something fishy going on, or as we say in Denmark, there are owls in the marsh. At least the plasmid seems correct.<br><br> | analysis: There realy is something fishy going on, or as we say in Denmark, there are owls in the marsh. At least the plasmid seems correct.<br><br> | ||
| + | |||
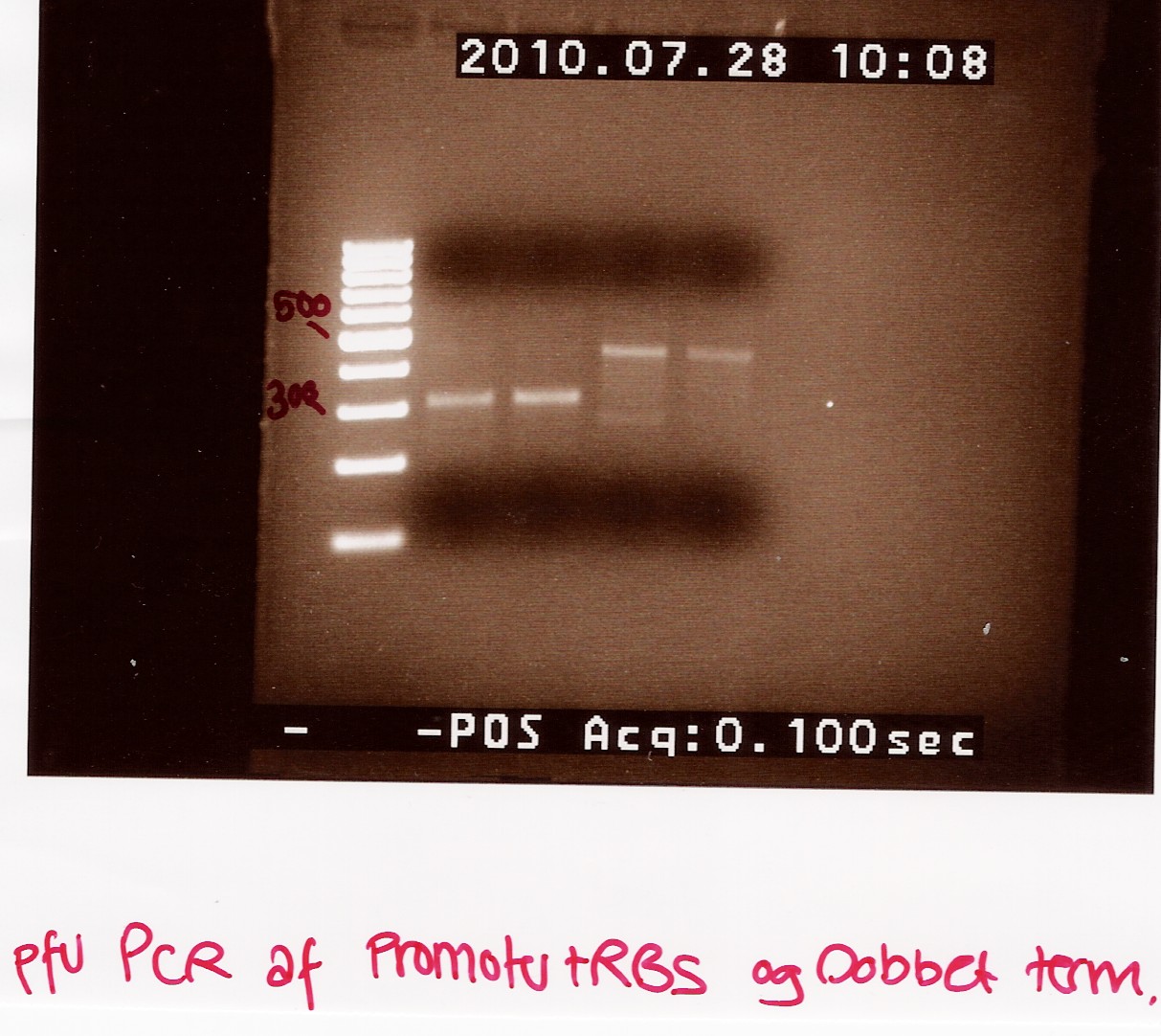
==== PCR on RD products from above experiments ==== | ==== PCR on RD products from above experiments ==== | ||
Date: 29/7<br> | Date: 29/7<br> | ||
| Line 1,290: | Line 1,283: | ||
We have succesfully shown that our primers will generate a band at our expected length, albeit a very weak one. Perhaps it takes some luck for the reaction to get started. We hope to repeat the experiment with pfu, to get a good product for ligation. We don't know if the cutting has made the difference between this round of pcr and the one performed a couple of days earlier, or if other factors, like the gradient have played in. | We have succesfully shown that our primers will generate a band at our expected length, albeit a very weak one. Perhaps it takes some luck for the reaction to get started. We hope to repeat the experiment with pfu, to get a good product for ligation. We don't know if the cutting has made the difference between this round of pcr and the one performed a couple of days earlier, or if other factors, like the gradient have played in. | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
== Group: Photosensor == | == Group: Photosensor == | ||
| Line 1,323: | Line 1,291: | ||
''Done by:'' Maria<br> | ''Done by:'' Maria<br> | ||
''Methods:'' pfu PCR, gel electrophoresis<br> | ''Methods:'' pfu PCR, gel electrophoresis<br> | ||
| - | + | protocols: [https://2010.igem.org/Team:SDU-Denmark/protocols#CP1.1 CP1.1]<br><br> | |
''Notes:''<br> | ''Notes:''<br> | ||
gel purified PCR product of BOO15 and J12003 (25 and 26 white) was used as template.<br> | gel purified PCR product of BOO15 and J12003 (25 and 26 white) was used as template.<br> | ||
| Line 1,469: | Line 1,437: | ||
''Results:''<br> | ''Results:''<br> | ||
gel electrophoresis:<br> | gel electrophoresis:<br> | ||
| - | [[Image:Team-SDU-Denmark-Amp.ofJ13002andB0015.jpg| | + | [[Image:Team-SDU-Denmark-Amp.ofJ13002andB0015.jpg|300px]]<br><br> |
''Analysis:''<br> | ''Analysis:''<br> | ||
The PCR product is OK and is used as template for pfu PCR.<br> | The PCR product is OK and is used as template for pfu PCR.<br> | ||
| Line 1,476: | Line 1,444: | ||
BOO15 is stored as 43 (white)<br> | BOO15 is stored as 43 (white)<br> | ||
--[[User:Tipi|Tipi]] 06:52, 29 July 2010 (UTC)<br><br> | --[[User:Tipi|Tipi]] 06:52, 29 July 2010 (UTC)<br><br> | ||
| + | |||
| + | === compent cell and transformation of photosensor and promotor+RNA in pSB3T5 === | ||
| + | <br> | ||
| + | ''Done by:'' Maria and Pernille <br> | ||
| + | ''Date:'' July 27th <br> | ||
| + | ''Protocol:'' [https://2010.igem.org/Team:SDU-Denmark/protocols#CC1.1 CC1.1] and [https://2010.igem.org/Team:SDU-Denmark/protocols#TR1.1 TR1.1]<br> | ||
| + | ''Notes:'' <br> | ||
| + | ''Results:'' The OD of the cells were measured every hour and the corelation was: | ||
| + | <table style="text-align: left; height: 198px; width: 154px;" border="1" | ||
| + | cellpadding="2" cellspacing="2"> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 67px;">Time /h<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 85px;">OD550 /Abs<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 67px;">1<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 85px;">0,024<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 67px;">2<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 85px;">0,048<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top; width: 67px;">3<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top; width: 85px;">0,116<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | <tr> | ||
| + | <td style="vertical-align: top;">4<br> | ||
| + | </td> | ||
| + | <td style="vertical-align: top;">0,58<br> | ||
| + | </td> | ||
| + | </tr> | ||
| + | </table> | ||
| + | <br><br> | ||
=== Coloni PCR of photosensor in pUC19 from transformation 27/7 === | === Coloni PCR of photosensor in pUC19 from transformation 27/7 === | ||
| Line 1,597: | Line 1,607: | ||
The PCR samples was loaded onto a 1% agarose gel. Gene ruler DNA ladder mix (red) was used as marker.<br><br> | The PCR samples was loaded onto a 1% agarose gel. Gene ruler DNA ladder mix (red) was used as marker.<br><br> | ||
''Results:''<br> | ''Results:''<br> | ||
| - | [[Image:Team-SDU-Denmark-coloniPCRPS.jpg| | + | [[Image:Team-SDU-Denmark-coloniPCRPS.jpg|300px]]<br><br> |
''Analysis:''<br> | ''Analysis:''<br> | ||
No bands are seen in any of the samples. This could be because the VF2 and VR primers does not anneal to the plasmid.<br> | No bands are seen in any of the samples. This could be because the VF2 and VR primers does not anneal to the plasmid.<br> | ||
| Line 1,722: | Line 1,732: | ||
PCR samples are loaded onto a 1% agarose gel. Gene ruler DNA ladder mix (red) is used as marker.<br><br> | PCR samples are loaded onto a 1% agarose gel. Gene ruler DNA ladder mix (red) is used as marker.<br><br> | ||
''Results:''<br> | ''Results:''<br> | ||
| - | [[Image:Team-SDU-Denmark-PSplasmid PCR.jpg| | + | [[Image:Team-SDU-Denmark-PSplasmid PCR.jpg|300px]]<br><br> |
''Analysis:''<br> | ''Analysis:''<br> | ||
Two weak bands at app. 4500bp and 850bp respectively. The upper band could correspond to the pKJ606 plasmid with insert, and the lower band might indicate VF2-VR without insert, rendering these results rather inconlclusive. Therefore an ON culture for miniprep was made. <br> | Two weak bands at app. 4500bp and 850bp respectively. The upper band could correspond to the pKJ606 plasmid with insert, and the lower band might indicate VF2-VR without insert, rendering these results rather inconlclusive. Therefore an ON culture for miniprep was made. <br> | ||
| Line 1,738: | Line 1,748: | ||
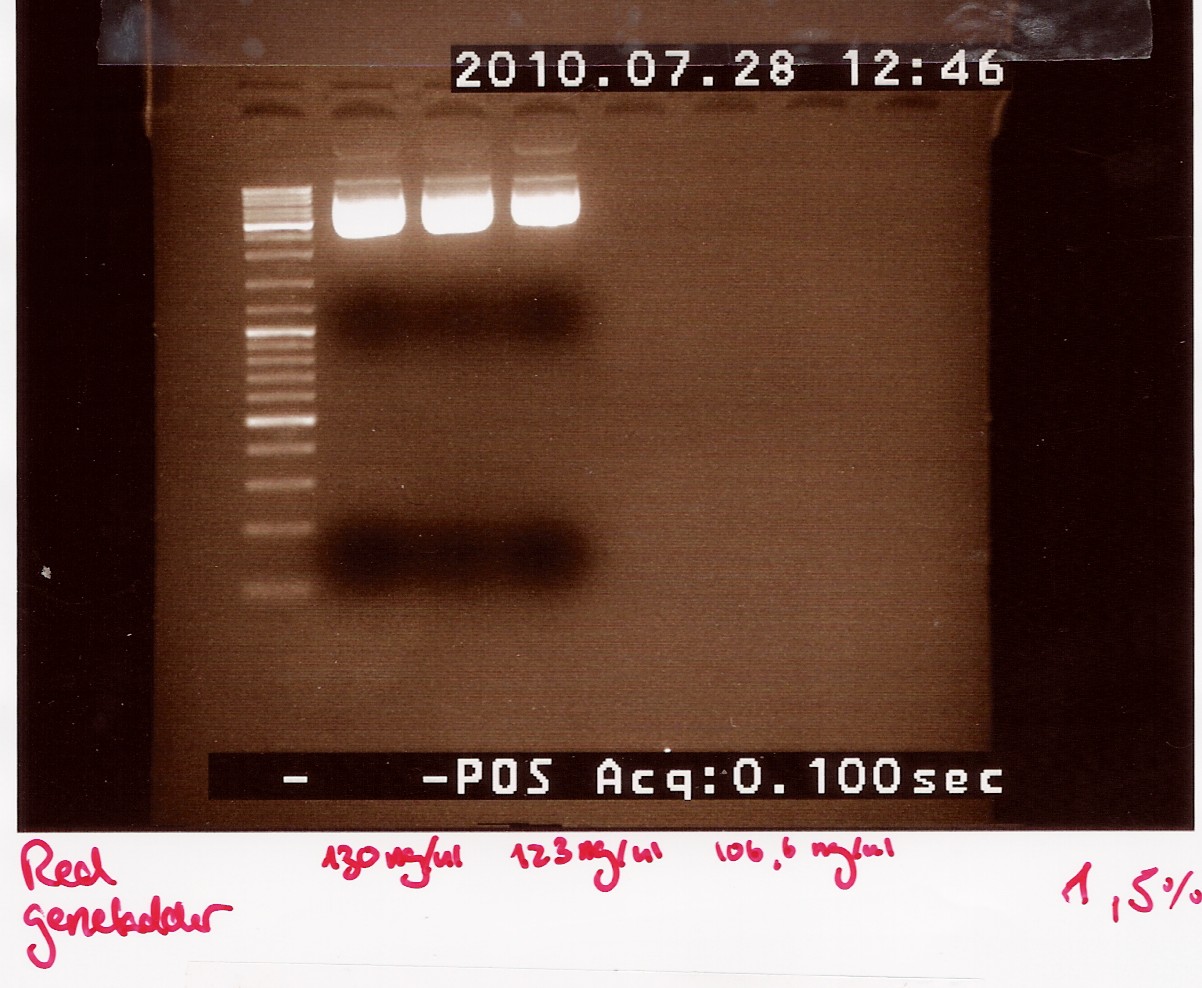
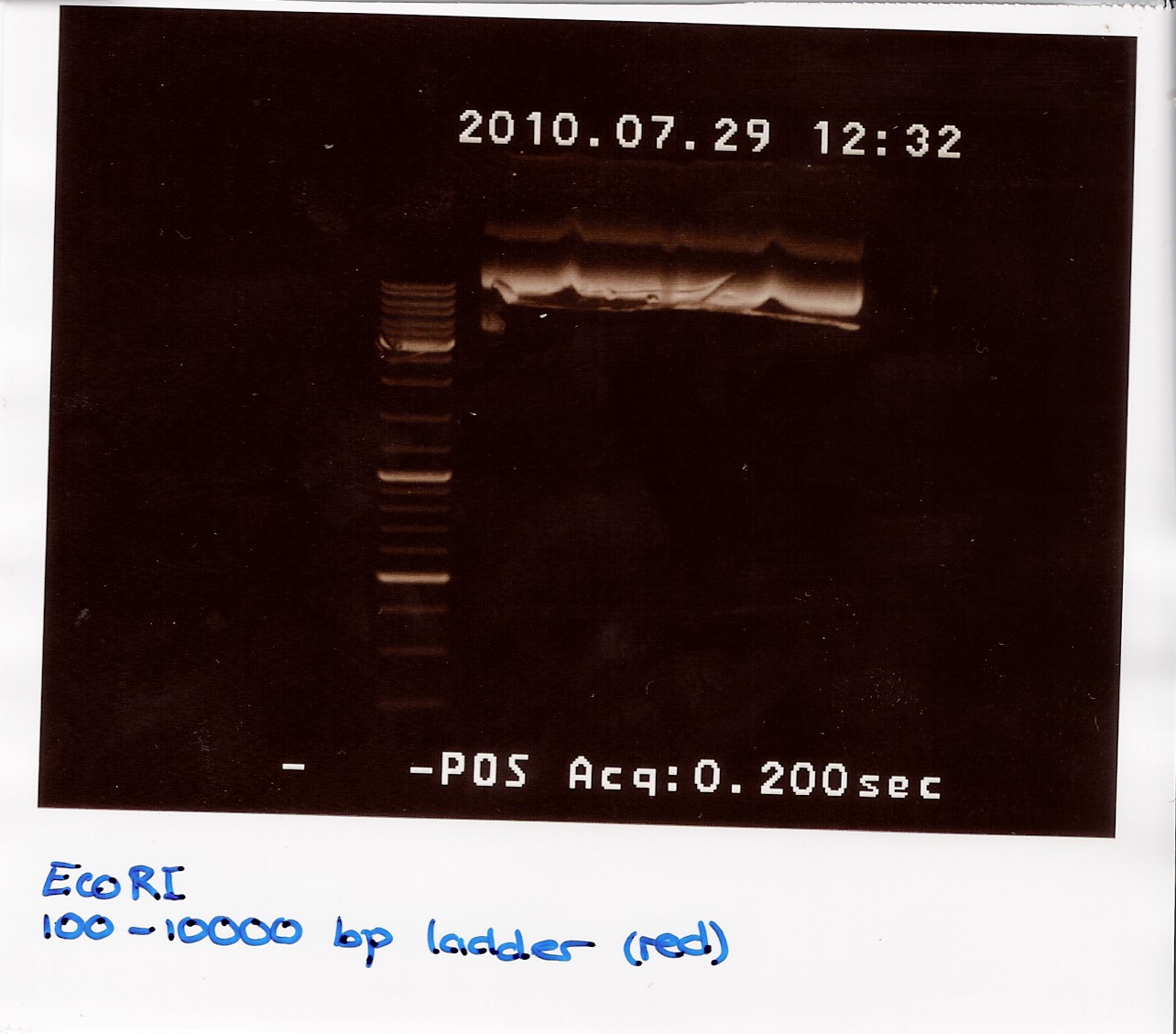
3: pOT2 with ''ninaB'' cut with EcoRI and XhoI (EX)<br> | 3: pOT2 with ''ninaB'' cut with EcoRI and XhoI (EX)<br> | ||
A 1.5% agarose gel was run:<br> | A 1.5% agarose gel was run:<br> | ||
| - | [[Image:team-sdu-denmark-NinaB_restriction_E.jpg | | + | [[Image:team-sdu-denmark-NinaB_restriction_E.jpg | 300px]] |
<br> | <br> | ||
Nucleic acid concentrations were measured with NanoDrop after gel extraction and purification: | Nucleic acid concentrations were measured with NanoDrop after gel extraction and purification: | ||
Latest revision as of 21:27, 23 October 2010
 "
"