Team:TU Munich/Modeling
From 2010.igem.org
Hartlmueller (Talk | contribs) |
Hartlmueller (Talk | contribs) |
||
| Line 19: | Line 19: | ||
=Diffusion= | =Diffusion= | ||
| - | |||
The question whether anti-termination occurs is not only guided by the folding process of the signal-terminator pair, but also by how long the signal takes to diffuse to the terminator sequence. | The question whether anti-termination occurs is not only guided by the folding process of the signal-terminator pair, but also by how long the signal takes to diffuse to the terminator sequence. | ||
| Line 46: | Line 45: | ||
As the folding time is significantly larger than the diffusion and thus much less relevant for modeling our signal-terminator constructs, we didn't employ more elaborate techniques to model diffusion. | As the folding time is significantly larger than the diffusion and thus much less relevant for modeling our signal-terminator constructs, we didn't employ more elaborate techniques to model diffusion. | ||
| - | + | ||
=Switch= | =Switch= | ||
| Line 55: | Line 54: | ||
| - | + | ||
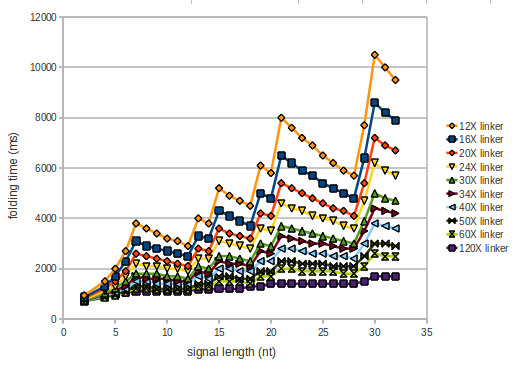
We modeled the signal-terminator interaction for 2, 4-32 nt signal length and proved that the signal sequence binds to the terminator sequence and impedes the terminator folding, thus the polymerase doesn't fall off and the output signal is produced. As we observed a dependence of the folding time and minimal energy respectively from the linker length we used linkers of length 12, 16, 20, 24, 30, 34, 40, 50, 60, 120 'X' bases. For the minimal energy there is only a very small linker length dependence as one can see in figure 1. | We modeled the signal-terminator interaction for 2, 4-32 nt signal length and proved that the signal sequence binds to the terminator sequence and impedes the terminator folding, thus the polymerase doesn't fall off and the output signal is produced. As we observed a dependence of the folding time and minimal energy respectively from the linker length we used linkers of length 12, 16, 20, 24, 30, 34, 40, 50, 60, 120 'X' bases. For the minimal energy there is only a very small linker length dependence as one can see in figure 1. | ||
[[Image:TU_Munich_iGEM2010_sstraub_folding_time.png]] | [[Image:TU_Munich_iGEM2010_sstraub_folding_time.png]] | ||
| Line 72: | Line 71: | ||
This video is done for full signal length of 32 nt. One can see that the terminator does not fold at all as the signal immediately binds to the terminator sequence as it is synthesized. | This video is done for full signal length of 32 nt. One can see that the terminator does not fold at all as the signal immediately binds to the terminator sequence as it is synthesized. | ||
| - | + | ||
==Trp-terminator== | ==Trp-terminator== | ||
Revision as of 09:50, 21 October 2010
|
||||||||
|
|
OverviewWe simulated the termination and anti-termination properties of our signal-terminator constructs with the Kinefold web server and used some standard estimations for diffusive terms. Our main goal was to prove that our constructs work and that termination is stopped efficiently, that is that the signal molecule binds and anti-terminations occurs before the RNA polymerase falls off.
DiffusionThe question whether anti-termination occurs is not only guided by the folding process of the signal-terminator pair, but also by how long the signal takes to diffuse to the terminator sequence.
To account for the diffusion time, we estimated the hit rate τ (following 6.), which is the time until the signal meets the terminator sequence for the first time: τ = 1/(3D*a/r3), where D is the diffusion constant, a the radius of gyration of the signal molecule and r the radius of the cell. a = (n*l)/3, where n is the length of the signal which is 0,3 nm/monomer, l is the persistency length which is following (5.) 2nm for single-stranded RNA. Thus, for a signal of length 32 nt, a = 6,4 nm. The diffusion constant D was obtained by D = kB T/ (6 π * 10*10-3*a), where kB is the Boltzmann constant and T is the absolute temperature. Thus, for a cell containing 100 signal molecules, the signal needs 0,1518 s until it first hits the terminator sequence. CloseAs the folding time is significantly larger than the diffusion and thus much less relevant for modeling our signal-terminator constructs, we didn't employ more elaborate techniques to model diffusion.
SwitchHis-terminatorSignal sequence: 5' GCGGGCUUUGCUGUUGUAGCAGGCGUCUUUGUUAGCUAGC 3'
We modeled the signal-terminator interaction for 2, 4-32 nt signal length and proved that the signal sequence binds to the terminator sequence and impedes the terminator folding, thus the polymerase doesn't fall off and the output signal is produced. As we observed a dependence of the folding time and minimal energy respectively from the linker length we used linkers of length 12, 16, 20, 24, 30, 34, 40, 50, 60, 120 'X' bases. For the minimal energy there is only a very small linker length dependence as one can see in figure 1.
RNA folding path videos:For this video the signal is only 2 nt long. One can see that the terminator is folding and that the signal cannot bind to the terminator sequence due to its short length. This video shows the folding of a terminator and a signal of length 20 nt. which shows that the terminator is already hindered from folding completely. This video is done for full signal length of 32 nt. One can see that the terminator does not fold at all as the signal immediately binds to the terminator sequence as it is synthesized.
Trp-terminatorResultsNetworkModelingResultsOutlook |
|||||||
 "
"