Team:Imperial College London/Modelling/Output/Detailed Description
From 2010.igem.org
(Updated the pictures) |
|||
| Line 1: | Line 1: | ||
{{:Team:Imperial_College_London/Templates/Header}} | {{:Team:Imperial_College_London/Templates/Header}} | ||
| + | {{:Team:Imperial_College_London/Templates/Buttons}} | ||
{| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:25px;" cellspacing="20" | {| style="width:900px;background:#f5f5f5;text-align:justify;font-family: helvetica, arial, sans-serif;color:#555555;margin-top:25px;" cellspacing="20" | ||
|- | |- | ||
| Line 13: | Line 14: | ||
<html><h3>1. Model based on Law of Mass Action</h3></html> | <html><h3>1. Model based on Law of Mass Action</h3></html> | ||
| - | During a meeting with our advisors, it was noted that our initial models (which had assumed that our system obeyed Michaelis Menten kinetics) were wrong | + | During a meeting with our advisors, it was noted that our initial models (which had assumed that our system obeyed Michaelis-Menten kinetics) were wrong as the assumptions made by Michaelis-Menten approximation were not obeyed by our system. Click on the button below to learn more about our models based on Michaelis-Menten kinetics. |
| + | <div class="accordionButton">Abandoned Initial Attempts</div> | ||
| + | <div class="accordionContent"> | ||
| + | <h2>Model based on Michaelis Menten Kinetics (Weeks 4 and 5)</h2> | ||
| + | <br/> | ||
| + | <b>HIV1</b><br/> | ||
| + | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" | ||
| + | | [[Image:Slide2.JPG|300px|thumb|center|alt=A|At each stage of amplification a distinct protease is being used ]] | ||
| + | | align="left"| | ||
| + | '''Equations''' | ||
| - | < | + | *<math>\dot{m}=k_{to} - d_{to}m\,\!</math> |
| - | In order to be able to use Michaelis Menten kinetics, there a lot of assumptions that have to be made. A few of these assumptions were not met by our system: | + | |
| + | *<math>\dot{p_h} = k_hm - d_hp_h</math> | ||
| + | |||
| + | |||
| + | *<math>\dot{p_t} = k_tp_h - d_tp_t</math> | ||
| + | |||
| + | |||
| + | *<math>\dot{p_g} = k_gp_t - d_gp_g</math> | ||
| + | | align="left"| | ||
| + | '''Parameters''' | ||
| + | *<math>k_{to}\mbox{...transcription rate of HIV1}</math> | ||
| + | *<math>d_{to}\mbox{...degradation rate of mRNA coding for HIV1}</math> | ||
| + | *<math>k_h\mbox{...translation rate of HIV1}</math> | ||
| + | *<math>d_h\mbox{...degradation rate of HIV1}</math> | ||
| + | *<math>k_t\mbox{...production rate of TEV by HIV1}</math> | ||
| + | *<math>d_t\mbox{...degradation rate of TEV}</math> | ||
| + | *<math>k_g\mbox{...production rate of GFP by TEB}</math> | ||
| + | *<math>d_g\mbox{...degradation rate of GFP}</math> | ||
| + | |} | ||
| + | <br/><br/> | ||
| + | <b>TEV</b><br/> | ||
| + | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" | ||
| + | |[[Image:TEV.jpg|300px|thumb|center|alt=A|TEV is used at both stages of amplification]] | ||
| + | |align="left"| | ||
| + | '''Equations''' | ||
| + | |||
| + | |||
| + | *<math>\dot{m} = k_{to} - d_{to}m</math> | ||
| + | |||
| + | |||
| + | *<math>\dot{p_t} = k_tm - d_tp_t</math> | ||
| + | |||
| + | |||
| + | *<math>\dot{p_{ts}} = k_{ts}p_t - d_{ts}p_{ts}</math> | ||
| + | |||
| + | |||
| + | *<math>\dot{p_g} = k_{g1}p_t + k_{g2}p_{ts} - d_gp_g</math> | ||
| + | |align="left"| | ||
| + | '''Parameters''' | ||
| + | *<math>k_{to}\mbox{...rate of transcription by TEV}</math> | ||
| + | *<math>d_{to}\mbox{...degradation rate of mRNA coding for TEV}</math> | ||
| + | *<math>k_t\mbox{...rate of translation of TEV}</math> | ||
| + | *<math>d_t\mbox{...degradation rate of TEV}</math> | ||
| + | *<math>k_{ts}\mbox{...rate of production (fusion) of split TEV}</math> | ||
| + | *<math>d_{ts}\mbox{...degradation rate of split TEV}</math> | ||
| + | *<math>k_{g1}\mbox{...rate of production of GFP by full TEV}</math> | ||
| + | *<math>k_{g2}\mbox{...rate of production of GFP by split TEV}</math> | ||
| + | *<math>d_g\mbox{...degradation rate of GFP}</math> | ||
| + | |} | ||
| + | |||
| + | <h2>Improved Model which accounts for enzyme reactions (28/07/2010)</h2> | ||
| + | <br/><br/> | ||
| + | <b>TEV</b><br/> | ||
| + | {| class="wikitable" style="text-align: center; width: 100%; height: 170px;" | ||
| + | |[[Image:TEV.jpg|500px|thumb|center|alt=A|TEV is used at both stages of amplification]] | ||
| + | |align="left"| | ||
| + | '''Equations''' | ||
| + | * 1. Production of TEV from transcription | ||
| + | <math>\dot{p_t} = s_t - d_tp_t</math> | ||
| + | |||
| + | <math>s_t = \dfrac{k_tk_{to}}{d_{to}}</math> | ||
| + | |||
| + | |||
| + | * 2. Production of split TEV from transcription | ||
| + | <math>\dot{p_{st}} = s_{st} - d_{st}p_{st}</math> | ||
| + | |||
| + | |||
| + | * 3. Production of split GFP from transcription | ||
| + | <math>\dot{p_{sg}} = s_{sg} - d_{sg}p_{sg}</math> | ||
| + | |||
| + | |||
| + | * 4. Production of fused split TEV catalysed by TEV (1) | ||
| + | <math>\dot{p_{ts}} = \dfrac{V_{max,t}[p_{st}]}{K_{m,ts} + [p_{st}]} - d_{ts}p_{ts}</math> | ||
| + | |||
| + | |||
| + | * 5. Production of GFP catalysed by TEV (1) and fused split TEV (4) | ||
| + | <math>\dot{p_g} = \dfrac{V_{max,tg}[p_{sg}]}{K_{m,tg} + [p_{sg}]} + \dfrac{V_{max,tsg}[p_{sg}]}{K_{m,tsg} + [p_{sg}]} - d_gp_g</math> | ||
| + | |} | ||
| + | |||
| + | <br/><br/> | ||
| + | <b>Implementation in Matlab</b><br/> | ||
| + | The Matlab code for the different stages of amplification and diagrams can be found [http://www.openwetware.org/wiki/Image:Modelling.docx here]. | ||
| + | <br/><br/> | ||
| + | <b>Kinetic constants</b><br/> | ||
| + | |||
| + | {| border="1" | ||
| + | ! | ||
| + | ! GFP | ||
| + | ! TEV | ||
| + | ! split TEV | ||
| + | ! split GFP | ||
| + | |- | ||
| + | |'''<math>Km</math> and <math>k_{cat}</math>''' | ||
| + | | - | ||
| + | |<math>K_m = 0.061</math>; <math>k_{cat} = 0.16</math>; [http://peds.oxfordjournals.org/cgi/reprint/14/12/993] | ||
| + | | 40% of value for TEV | ||
| + | | - | ||
| + | |- | ||
| + | | '''Half-life or degradation rate''' | ||
| + | | Half-life in B.sub approximately 1.5 hours | ||
| + | | ? | ||
| + | | ? | ||
| + | | Half-life shorter than GFP | ||
| + | |- | ||
| + | | '''Production rate in B.sub''' | ||
| + | | ? | ||
| + | | ? | ||
| + | | ? | ||
| + | | ? | ||
| + | |} | ||
| + | |||
| + | <h2>Conclusion</h2> | ||
| + | |||
| + | We were not able to obtain all the necessary constants. Hence, we decided to make educated guesses about possible relative values between the constants as well as varying them and observing the change in output. | ||
| + | |||
| + | As the result, we concluded that the amplification happens at each amplification level proposed. The magnitude of amplification varies depending on the constants. There is not much difference between using TEV or HIV1. | ||
| + | |||
| + | <h2>References</h2> | ||
| + | #Kapust R. et al (2001) Tobacco etch virus protease: mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Protein Engineering. [Online] 14(12), 993-1000. Available from: http://peds.oxfordjournals.org/cgi/reprint/14/12/993 [Accessed 28th July 2010] | ||
| + | </div><br/><br/><br/> | ||
| + | In order to be able to use Michaelis Menten kinetics, there a lot of assumptions that have to be made. A few of these assumptions were not met by our system: | ||
*'''Vmax is proportional to the overall concentration of the enzyme.''' | *'''Vmax is proportional to the overall concentration of the enzyme.''' | ||
Since we are continuously producing enzyme, Vmax will change. Therefore the conservation <html>E<sub>0</sub> = E + E<sub>S</sub></html> does not hold for our system. | Since we are continuously producing enzyme, Vmax will change. Therefore the conservation <html>E<sub>0</sub> = E + E<sub>S</sub></html> does not hold for our system. | ||
| Line 32: | Line 162: | ||
<html><h3>2. Model preA: Simple production of dioxygenase</h3></html> | <html><h3>2. Model preA: Simple production of dioxygenase</h3></html> | ||
| - | [[Image:IC_Simple_production.JPG|300px| | + | <div ALIGN=CENTER> |
| + | {| style="background:#e1e1e1;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:IC_Simple_production.JPG|300px]] | ||
| + | |- | ||
| + | |Transcription and translation<br/>(simple production) of dioxygenase. | ||
| + | |} | ||
| + | </div> | ||
This model includes transcription and translation of the dioxygenase. It does not involve any amplification steps. It is our control model against which we will be comparing the results of other models. | This model includes transcription and translation of the dioxygenase. It does not involve any amplification steps. It is our control model against which we will be comparing the results of other models. | ||
| Line 38: | Line 175: | ||
<html><h3>3. Model A: Activation of Dioxygenase by TEV enzyme</h3></html> | <html><h3>3. Model A: Activation of Dioxygenase by TEV enzyme</h3></html> | ||
| - | [[Image:IC_1-step_amplification.JPG|300px| | + | <div ALIGN=CENTER> |
| + | {| style="background:#e1e1e1;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:IC_1-step_amplification.JPG|300px]] | ||
| + | |- | ||
| + | |1-step amplification. | ||
| + | |} | ||
| + | </div> | ||
| - | |||
| - | + | This model consists of the basic enzymatic reaction: | |
| + | <br/><html> | ||
| + | <CENTER><img src="https://static.igem.org/mediawiki/2010/7/7a/Equations1.png"alt="Equations showing enzymatic reaction between TEV and split Dioxygenase" /></CENTER> | ||
| + | </html> | ||
This is a simple enzymatic reaction, where TEV is the enzyme, Dioxygenase the product and split Dioxygenase the substrate. Choosing <html>k<sub>1</sub>, k<sub>2</sub>, k<sub>3</sub></html> as reaction constants, the reaction can be rewritten in these four sub-equations: | This is a simple enzymatic reaction, where TEV is the enzyme, Dioxygenase the product and split Dioxygenase the substrate. Choosing <html>k<sub>1</sub>, k<sub>2</sub>, k<sub>3</sub></html> as reaction constants, the reaction can be rewritten in these four sub-equations: | ||
| - | + | <br/><html> | |
| - | + | <CENTER><img src="https://static.igem.org/mediawiki/2010/a/a5/Equations2.png"alt="PDEs describing the reaction presented above"/></CENTER> | |
| + | </html> | ||
These four equations were implemented in Matlab, using a built-in function (ode45) which solves ordinary differential equations. | These four equations were implemented in Matlab, using a built-in function (ode45) which solves ordinary differential equations. | ||
| Line 54: | Line 201: | ||
Another approach to model the amplification module would be to implement it in a program such as TinkerCell (or CellDesigner). This would be useful to check whether the Matlab model works. | Another approach to model the amplification module would be to implement it in a program such as TinkerCell (or CellDesigner). This would be useful to check whether the Matlab model works. | ||
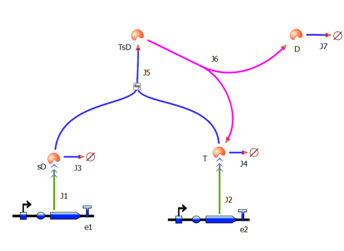
| - | [[Image:IC_Tinkercell_Model_A.PNG|300px| | + | <div ALIGN=CENTER> |
| - | + | {| style="background:#e1e1e1;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;margin- top:5px;padding: 2px;" cellspacing="5"; | |
| + | |- | ||
| + | |[[Image:IC_Tinkercell_Model_A.PNG|300px]] | ||
| + | |- | ||
| + | |Model A implemented in TinkerCell. | ||
| + | |} | ||
| + | </div> | ||
<html><h3>4. Model B: Activation of Dioxygenase by TEV or activated split TEV enzyme</h3></html> | <html><h3>4. Model B: Activation of Dioxygenase by TEV or activated split TEV enzyme</h3></html> | ||
| - | [[Image:IC_2-step_amplification.JPG|300px| | + | <div ALIGN=CENTER> |
| + | {| style="background:#e1e1e1;text-align:center;font-family: helvetica, arial, sans-serif;color:#555555;margin- top:5px;padding: 2px;" cellspacing="5"; | ||
| + | |- | ||
| + | |[[Image:IC_2-step_amplification.JPG|300px]] | ||
| + | |- | ||
| + | |2-step amplification. | ||
| + | |} | ||
| + | </div> | ||
This version includes the following features: | This version includes the following features: | ||
Revision as of 16:48, 20 October 2010
| Temporary sub-menu: Objectives; Detailed Description; Parameters & Constants; Results & Conclusion;Download MatLab Files; |
| Output Amplification Model | |||||||||||||||||||||||||||||
Detailed Description1. Model based on Law of Mass ActionDuring a meeting with our advisors, it was noted that our initial models (which had assumed that our system obeyed Michaelis-Menten kinetics) were wrong as the assumptions made by Michaelis-Menten approximation were not obeyed by our system. Click on the button below to learn more about our models based on Michaelis-Menten kinetics. Abandoned Initial Attempts
Model based on Michaelis Menten Kinetics (Weeks 4 and 5)
Improved Model which accounts for enzyme reactions (28/07/2010)
ConclusionWe were not able to obtain all the necessary constants. Hence, we decided to make educated guesses about possible relative values between the constants as well as varying them and observing the change in output. As the result, we concluded that the amplification happens at each amplification level proposed. The magnitude of amplification varies depending on the constants. There is not much difference between using TEV or HIV1. References
In order to be able to use Michaelis Menten kinetics, there a lot of assumptions that have to be made. A few of these assumptions were not met by our system:
Since we are continuously producing enzyme, Vmax will change. Therefore the conservation E0 = E + ES does not hold for our system.
We are producing both substrate and enzyme, so we have approximately the same amount of substrate and enzyme.
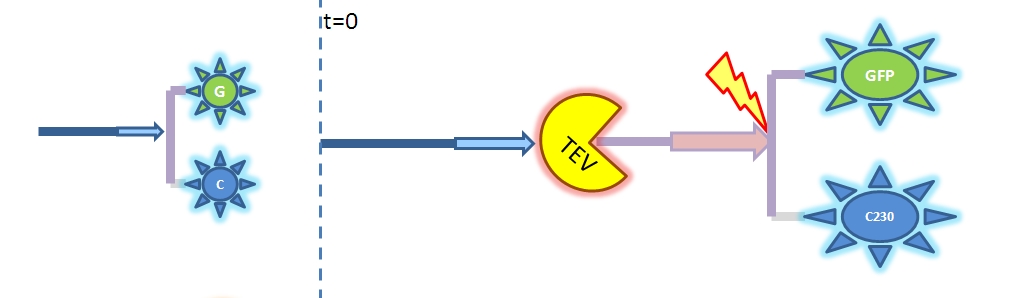
Therefore, the model above is not representative of the enzymatic reaction. As we cannot use the Michaelis-Menten model we will have to solve from first principle (which involves writing down all of the biochemical equations and solving for these in Matlab). Change of output During our literature research, we came across a better output, so we abandoned the idea of using GFP as an output. Instead, we are using catechol. An enzyme, dioxygenase, will be acting on the catechol, which will then result in a coloured output. Catechol will be added to the bacteria manually (i.e. the bacteria will not produce catechol). Hence, in our models dioxygenase will be treated as an output as this enzyme is the only activator of catechol in our system. This means that the change of catechol into its colourful form is dependent on the dioxygenase concentration.
2. Model preA: Simple production of dioxygenaseThis model includes transcription and translation of the dioxygenase. It does not involve any amplification steps. It is our control model against which we will be comparing the results of other models. 3. Model A: Activation of Dioxygenase by TEV enzyme
 This is a simple enzymatic reaction, where TEV is the enzyme, Dioxygenase the product and split Dioxygenase the substrate. Choosing k1, k2, k3 as reaction constants, the reaction can be rewritten in these four sub-equations:
 These four equations were implemented in Matlab, using a built-in function (ode45) which solves ordinary differential equations. Implementation in TinkerCell Another approach to model the amplification module would be to implement it in a program such as TinkerCell (or CellDesigner). This would be useful to check whether the Matlab model works. 4. Model B: Activation of Dioxygenase by TEV or activated split TEV enzymeThis version includes the following features:
5. Model C: Further improvementsThis model has not been implemented because of the conclusions that we reached from Models A and B. It would include the following features:
|
 "
"