Team:TU Munich/Lab
From 2010.igem.org
(→Experiment Design) |
|||
| Line 5: | Line 5: | ||
=Experiment Design= | =Experiment Design= | ||
| - | We designed different experiment set-ups with different complexity to test RNA signal/switch pairs | + | We designed different experiment set-ups with different complexity to test RNA signal/switch pairs based on our concept.<br> |
Our initial idea to prove our concept of antitermination was to use flourescent proteins as reporters. This approach gives the opportunity to measure the termination and antitermination efficiency of our designed BioBricks ''in vivo'' as well as ''in vitro'', the latter using a translation kit based on <i>E. coli</i> lysate. Later on, we decided to developed an experiment, that relies only on transcription. In this, we used a fluorescent dye, malachite green, that bind a specific RNA aptamer and thus makes it possible to detect transcription activity.<br> | Our initial idea to prove our concept of antitermination was to use flourescent proteins as reporters. This approach gives the opportunity to measure the termination and antitermination efficiency of our designed BioBricks ''in vivo'' as well as ''in vitro'', the latter using a translation kit based on <i>E. coli</i> lysate. Later on, we decided to developed an experiment, that relies only on transcription. In this, we used a fluorescent dye, malachite green, that bind a specific RNA aptamer and thus makes it possible to detect transcription activity.<br> | ||
==''In vivo'' Measurements== | ==''In vivo'' Measurements== | ||
Revision as of 15:33, 17 October 2010
|
||||||||
|
|
Experiment DesignWe designed different experiment set-ups with different complexity to test RNA signal/switch pairs based on our concept. In vivo Measurements
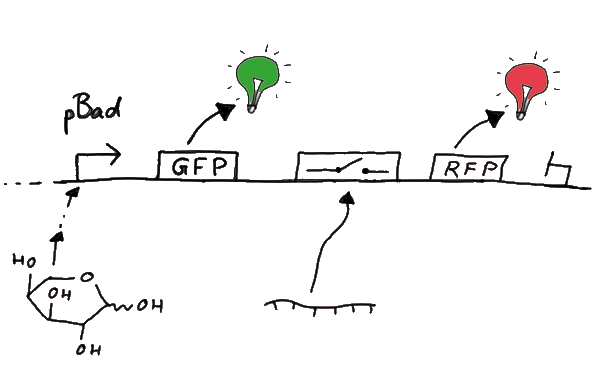
In principle we decided to use an expression cassette consisting of Green Fluorescent Protein (GFP) coding sequence upstream of the switch and another fluorescent protein coding sequence downstream of it. Both protein coding seuqence carry the same ribosome binding site, therefore, the GFP fluorescence can be used as internal control in measurements. Since the spectra should not overlap and to avoid FRET as well as an pure overlap of the spectra, we settled on the usage of red fluorescent protein variants, namely mRFP1 in the first try. While the GFP fluorescence is used to normalize the measurements, the RFP fluorescence is used to detect termination/antitermination.
Upon binding of the signal the stem loop of the switch would resolve leading to red fluorescent additional to the green one of GFP. The internal control carries the advantage that errors in the measurement set can be detected easily. Lack of arabinose or promotor insensitivity can be recognized as well as problems with the fluorescence measurement itself. Plus, we have a way to normalize our measurements and compare different preparations in relation to each other.
Our measuring plasmid is based on the BioBrick pSB1A10, A1, distribution 2010. Unfortunately after two months of cloning we had to recognize that the plasmid in use did not work (see also Biobrick validation--> link). So after the first unsuccessful attempts we had to reclone the system, substituing RFP to mCherry, a dsRED derivative with a spectra in the far red, and adding arabinose inducible promoters in front of both fluorescent proteins.
For switch evaluation, IPTG was added to the cells after about two hours after arabinose induction (baseline). ??? Stimmt das?? A rise of RFP/mCherry emission should be visible in case of a working switch.
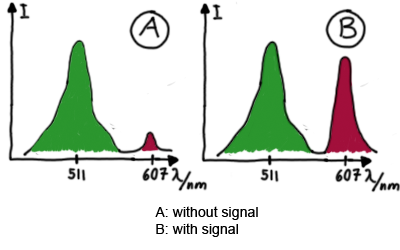
When measuring the termination of our BioBricks and the antitermination by their corresponding signal-RNA, we should be able to observe an increasing RFP emission compared to the GFP emission upon induced signal-RNA production in the cells/in the kit:
With these measurements, it should also be possible to observe differences in efficiency of termination as well as antitermination between our designed switches. In vitro Translation
So haben wir in vivo translation gemessen CloseIn vitro Transcription
Since our switch is RNA-based and the whole mechanism takes place on trancriptional level, we looked for a method to check for termination efficiency only on RNA-level. In vitro transcription offers an elegant way for a fast and easyn prove of principle. If measureable effects with our basic concept can be seen in vitro we can use the so gained data to optimize the system in vivo. Since we are working on a totally new principle of trancriptional control, we used this approach for easy variation of different variables like the length of the core unit and the switch to signal ratio.
To study the switches on the transcriptional level gives the advantage, that we would have less interferences and possible artefacts. Also, we are not sure how cellular mechanisms like degradation of RNases or interacting factors as well as molecular crowding influence our systems.
T7 RNA polymeraseThe T7 RNA polymerase is known for satisfying RNA yields together with easy handling. In our approach we had PCR amplified, double stranded switches with an malachitegreen binding aptamer following after the switch (133 bp, see section below) and a single stranded signal with about 30 bp length.
For in vitro expression the T7 RNA Polymerase requires a double stranded promotor region at the beginning of the DNA template but is otherwise capable of handling single stranded DNA, so a sense strain corresponding to the T7 promoter region was added. Transcription is more effective with double stranded DNA as template. Since we ordered the signal sequences we tested we chose the cheaper way in the beginning by using single stranded signals with corresponding sense T7 pieces and switched to double stranded constructs after narrowing down the most promising switch/signal pairs. E. coli RNA polymeraseDenaturing Polyacrylamide gel electrophoresisWe also used Polyacrylamide gel electrophoresis (PAGE) for evaluation of termination efficiency of our basic units. Gels containing 15 % acrylamide and 6 M urea were used for separation of 90 (terminated by switch) and 133 bp (continous reading) RNAs.
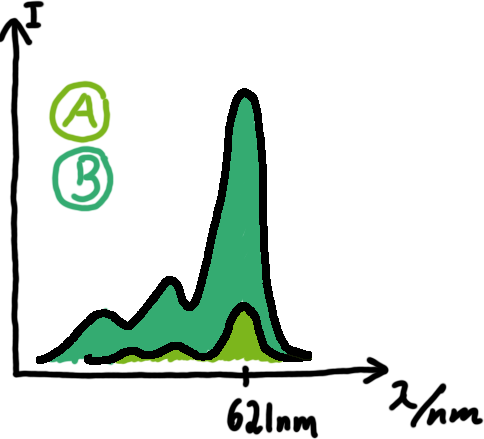
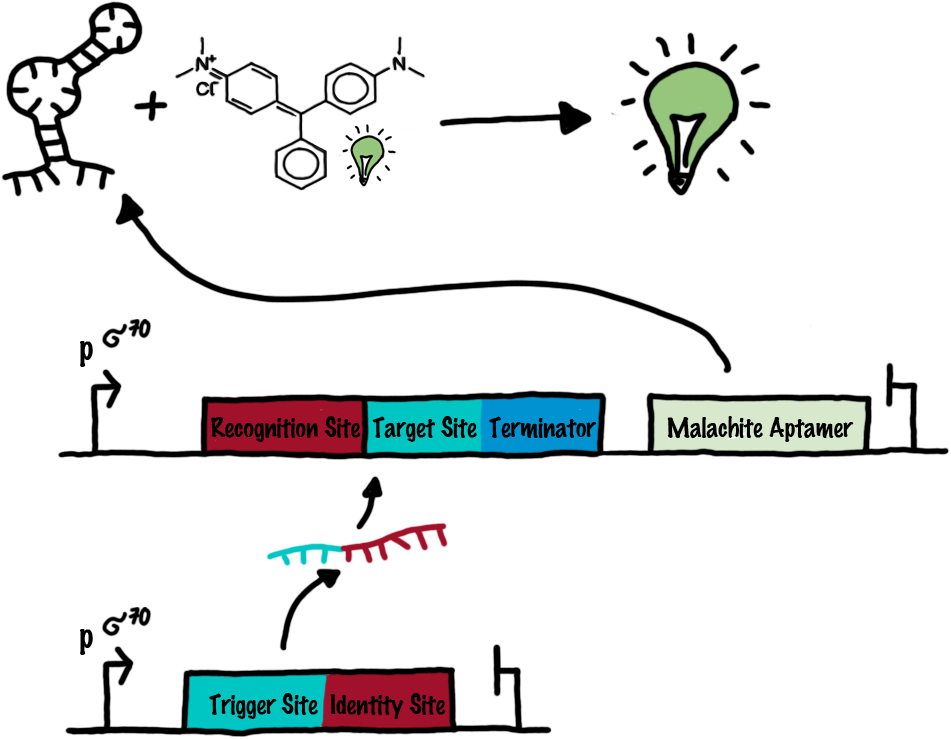
Malachite green assayMalachite-green is a dye with a negligible fluorescence in solution but undergoes a dramatic increase if bound by a RNA -aptamer. Upon binding to the aptamer, the fluorescence of malachite-green increases about 3000 times making it an exceptionel good marker. Since the binding is very specific, transcription in dependence of a signal can be monitored by measuring the fluorescence of malachite-green over time if the aptamer is located behind the switch. Transcription of the aptamer will only take place after anti-termination by a signal. An increase should be visible over time.
OLD: A second possibility to measure parameters of our switches we came up with, was the idea to investigate our system on the transcriptional level only. Therefore, we decided to use malachite green as reporter. Malachite green in a fluorescent dye, whose emission increasing dramaticly (about 3000 times) upon binding of a specific RNA-aptamer. The RNA-aptamer
We made constructs comprising of a sigma(70)-binding promoter followed by a short nonsense sequence, the switches and the aptamer sequence.
Close Close ProtocolsMolecular BiologyPCR
So geht ne PCR CloseDigestion
So geht ne PCR CloseLigation
In vivo measurement
So haben wir in vivo gemessen CloseIn vitro expression
So haben wir in vitro gemessen CloseIn vitro transcription
So haben wir in vivo gemessen Close
Lab BookExplanation:
|
|||||||
 "
"