Team:Uppsala-SwedenWeek9
From 2010.igem.org



Week-9
Identification of Bio-bricks
Plasmid of the samples which showed right size band on the gel were sent to a plasmid concentration test. We decided only F2, F6, F7, F8, F15 and F16 were in good concentration to proceed.
|
Sample |
A[260] |
A[280] |
Bg[320] |
Ratio |
Protein (μg/ml) |
Nucleic Acid (μg/ml) |
|
f2 |
0,0946 |
0,0607 |
0,0202 |
|
|
|
|
f3 |
0,0444 |
0,0346 |
0,0216 |
1,7474 |
2,9830 |
0,9640 |
|
f6 |
0,0844 |
0,0669 |
0,0300 |
1,4721 |
16,1731 |
2,0934 |
|
f7 |
0,1348 |
0,1020 |
0,0557 |
1,7074 |
11,9965 |
3,3075 |
|
f8 |
0,0905 |
0,0690 |
0,0420 |
1,7961 |
5,1758 |
2,0767 |
|
f11 |
0,0332 |
0,0261 |
0,0184 |
1,9261 |
0,7189 |
0,6559 |
|
f15 |
0,0769 |
0,0511 |
0,0205 |
1,8439 |
4,7573 |
2,4456 |
|
f16 |
0,0543 |
0,0389 |
0,0223 |
1,9290 |
1,5147 |
1,4182 |
As the first PCR didn't show the length of all biobricks, the samples failed last time were sent to run another c-PCR. The result was showed in Fig.1
However, only sample F11 shows the band with right size on the gel pic. Samples, F1, F3, F4, F5, F9, F10, F11, F12, F13, F14 and F17 were inoculated again to get enough plasmid for further use. Second colony PCR was performed again for all the starting biobricks and the products were run for gel again. Comparing the DNA length to the band size, all the biobricks were confirmed, except for F16.
The concentration of plasmid were measured for all the samples and values were showed in Table 2
Analysis
Collection time 2010-08-05 14:34:11
Sample A[260] A[280] Bg[320] Ratio Protein Nucleic Acid
µg/ml µg/ml
____________________________________________________________________________________
f1 0,0183 0,0101 -0,0027 1,6428 3,9441 86,2531
f2 0,0477 0,0334 0,0160 1,8282 2,9083 137,1389
f3 0,0255 0,0132 -0,0021 1,8045 2,8374 118,5919
f4 0,0518 0,0398 0,0216 1,6573 5,4159 124,4569
f5 0,0108 0,0033 -0,0041 2,0017 0,2684 66,7742
f6 0,0137 0,0079 0,0006 1,7979 1,3839 56,0149
f7 0,0072 0,0010 -0,0071 1,7638 1,7529 60,7466
f8 -0,0035 -0,0080 -0,0151 1,6180 2,3362 47,0326
f9 0,0104 0,0035 -0,0054 1,7835 1,7833 67,4654
f10 0,0074 -0,0004 -0,0076 2,0846 -0,1927 68,6519
f11 0,0184 0,0088 -0,0063 1,6384 4,6986 101,2263
f12 0,0008 -0,0044 -0,0115 1,7349 1,6832 51,6802
f13 0,0157 0,0066 -0,0074 1,6499 4,2257 94,6587
f14 0,0033 -0,0031 -0,0100 1,9215 0,6691 58,6173
f15 0,0403 0,0345 0,0294 2,1530 -0,3966 50,2377
16 0,0182 0,0075 -0,0052 1,8335 2,0872 101,2592
f17 0,0082 0,0036 -0,0020 1,8332 0,9140 44,2838
Concentration before dilution for dection
ng/µL
f1 52
f2 82
f3 71
f4 75
f5 40
f6 34
f7 36
f8 28
f9 40
f10 41
f11 61
f12 31
f13 57
f14 35
f15 30
f16 61
F17 27
Plasmid of F1, F2, F4, F9,F10,F12, F17,ccdb C3 and ccdb K3 were digested with proper enzymes bought from Sigma-Aldrich. The biobricks were ligated in pairs according to the plan by Quick Ligase kit from Fermentas. The ligation product were transformed into DH5α competent cells and each sample were plated on the agar plate which antibiotics matched to its backbone antibiotic resistance.
Construction of G1- G7
5 colonies were picked from each of those plates and sent to perform c-PCR. According to the band size on gel picture, 1 or 2 colonies of each set were selected to inoculate.
Again, inoculated samples were sent to perform plasmid extraction and measure the plasmid concentration.
Table . Plasmid concentration of G sets and F16.
Plasmids were digested with proper enzymes bought from Sigma-Aldrich and run for gel to confirm the size.
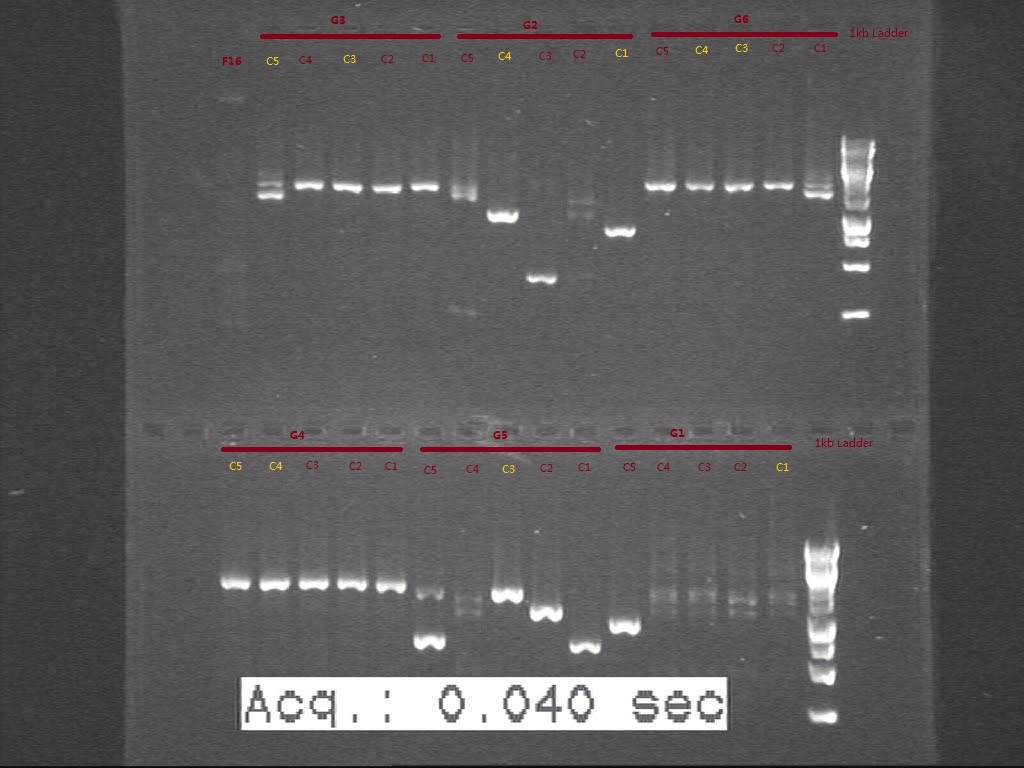
Figure . Gel picture shows the length of digested G sets.
From the gel, we think G1 and G2C4 seems having the right biobricks in size. G5 seems not digested well, though the plasmid seems in right size. The colonies we picked for G3, G4, G6 seems in wrong size according to c-PCR. Another transformation is necessary for G3, G4, and G6 and the starting biobricks need to be checked again by gel.
Figure . Gel result to verify length of G5C3 and the starting material of G3, G4, and G6
All the starting material of G3, G4, and G6 seems good, though G5 C3 doesn’t seems in the right length. So a second transformation was done for G3, G4, G5, G6. After transformation, the cells were plated on the proper antibiotic plates. 12 colonies were picked from each plates and sent to perform c-PCR. The c-PCR result was shown on the gel picture.
Figure Gel picture shows the c-PCR result of second time transformation product of G3. The samples marked in orange were thought in right size and proceed to inoculate. Figure Gel picture shows the c-PCR result of second time transformation product of G4. The samples marked in orange were thought in right size and proceed to inoculate. Figure Gel picture shows the c-PCR result of second time transformation product of G5. The samples marked in orange were thought in right size and proceed to inoculate. Figure Gel picture shows the c-PCR result of second time transformation product of G6. The samples marked in orange were thought in right size and proceed to inoculate.
Selected colonies were inoculated and performed plasmid extraction on the second day. The plasmid concentration was measured and result is showed in Table 4
Table . Plasmid concentration of G3, G4, G6 sets
For further confirm the construction of G sets, we decided to use some specific digestion and run the gel to verify the special sites on each construct. The digestion enzymes were chosen by using Gentle.
Table . Specific enzymes chosen for each construct and the expected length of all digested parts Figure . Gel picture shows the result of specific digestion of G3, G4, G5 and G6.
Only samples from G4 and G6 showed in the expected length and consistence. A second specific digestion were performed to samples of G3 and G5
Figure . Gel picture shows the result of specifc digestion of G3 and G5
Comparing the expected length of digested parts, G3C5, G4C6, G5C12 and G6C6 were digested and ligated according to the plan
 "
"