Team:Uppsala-SwedenWeek16
From 2010.igem.org
(→Week-16) |
(→Week-16) |
||
| Line 317: | Line 317: | ||
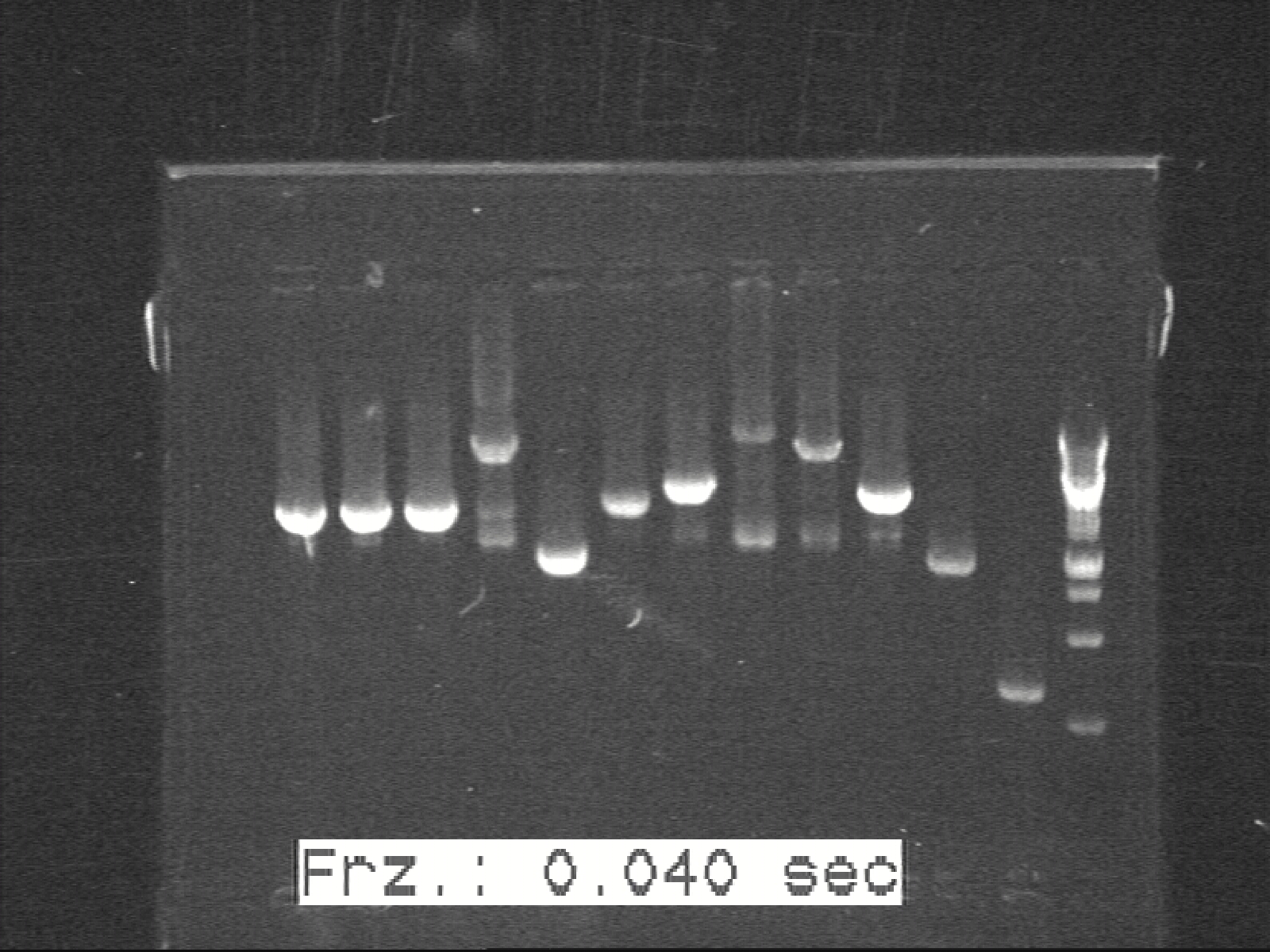
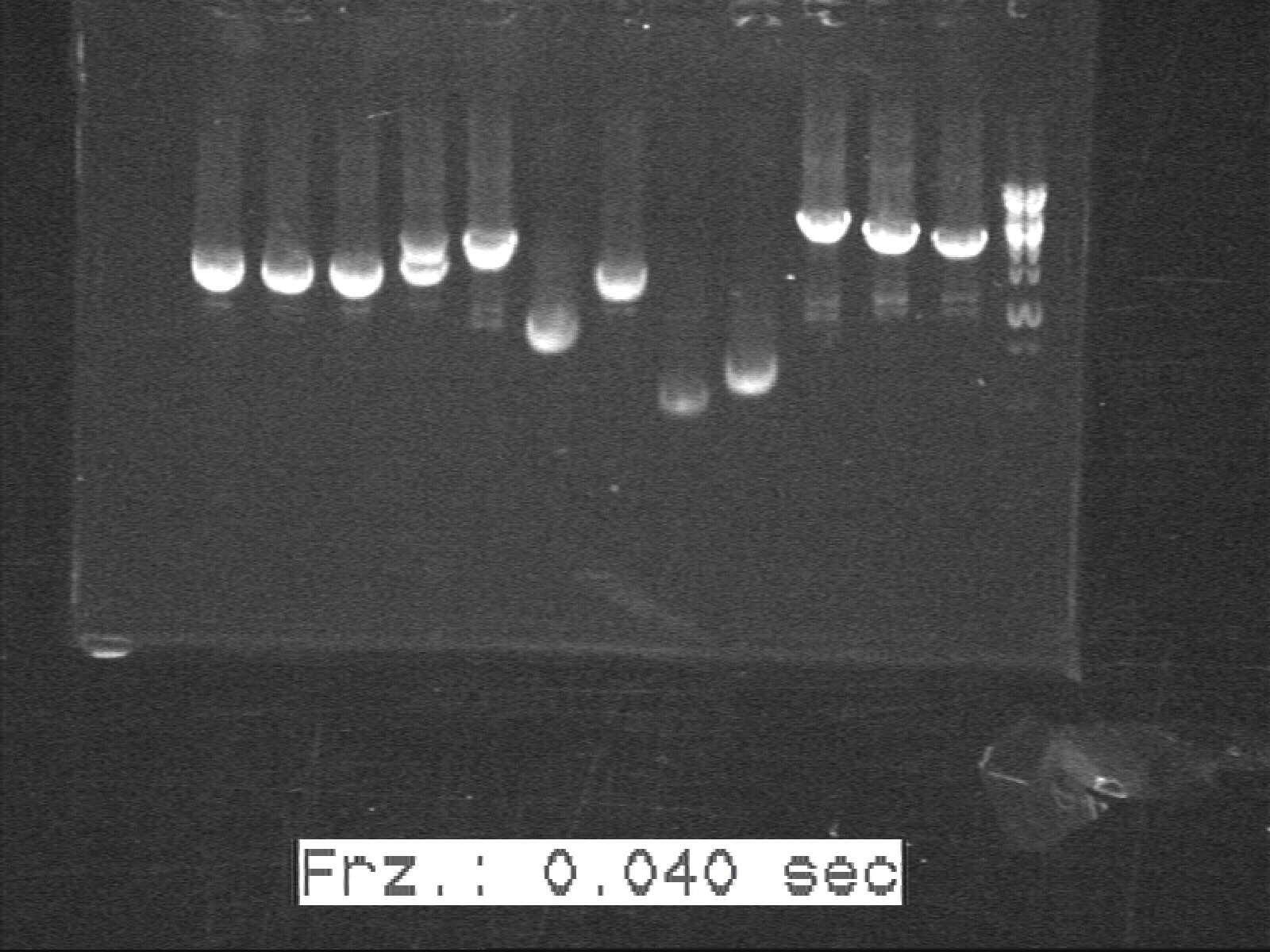
Phusion colony-PCR for K1, K2, K3, G1, G2 with Phusion Polymerase | Phusion colony-PCR for K1, K2, K3, G1, G2 with Phusion Polymerase | ||
| - | [[ | + | [[Image:Labnotes html m6b5aec6d.jpg|300px|thumb|left|K1 K2 K3 G1 G2]] |
[[Image:Labnotes html 42a7cca1.jpg|300px|thumb|K1 K2 K3 G1 G2]] | [[Image:Labnotes html 42a7cca1.jpg|300px|thumb|K1 K2 K3 G1 G2]] | ||
Revision as of 19:48, 26 October 2010



Week-16
Start to apply high fidelity PCR due the construct are getting larger and the regular Taq polymerase may not perform as well as the Phusion Polymerase at this length. Based on the company´s manual, we calculated the
|
|
Temperatur |
Time |
Cycle |
|
Initial denaturation |
98 |
30 s |
1 |
|
Denaturation |
98 |
10 |
30 |
|
Annealing |
55.3 |
10s |
30 |
|
Extension |
72 |
1 min 45 s |
30 |
|
Final extension |
72 |
5 min |
1 |
|
|
4 |
forever |
|
The purpose of this was to get more reliable band when doing a PCR and running the gel.
|
Mastermix |
Volume (µL) |
|
H20 |
10.8 |
|
5X phusion HF Buffer |
4 |
|
dNTPs |
2 |
|
VF2 |
1 |
|
VR |
1 |
|
DNA-template |
1 |
|
Phusion Polymerase |
0.2 |
J1C3, J2C3 and J3C1 was picked to build the K4,K5,K6 based on the gelpictures from 27/8/2010. Therefore we do not proceed with the ligated J-construct from the 16/9 due we have good enough samples from August month
Concentration measurement and swithch the backbone from pSB1C3 to psB1A3
|
|
DNA-conc.
|
DNA-vol Digestion |
Restr.Enzym E & P |
FD Buffer 10x |
H20 |
Total |
|
J1C3 |
97.2 |
10.3 |
0.5 & 0.5 |
2 |
6.7 |
20 |
|
J2C3 |
73.6 |
13.6 |
0.5 & 0.5 |
2 |
3.4 |
20 |
|
J3C1 |
71.2 |
14.0 |
0.5 & 0.5 |
2 |
3 |
20 |
|
ccdbA3 |
180.0 |
5 |
0.5 & 0.5 |
2 |
12 |
20 |
Ligation and transformation according the protocol
Phusion colony-PCR for K1, K2, K3, G1, G2 with Phusion Polymerase
We had unsatisfied results with our colonies fluorescence measurement and we suspected that the LacI protein didn’t suppress the fluorescent protein enough. One possible explanation would that BB_P0412 contains BB_C0012 which is the coding region for the LacI protein were degraded too effectively by the LVA degradation tail. As a consequence the R0010, a LacI promoter suppressing the three plasmids flourescent expression, didn’t bind enough LacI and therefore you could detect some flourescence.
Since J-construct contains the BB_R0010 as well, the K4, K5 and K6 construction were postponed.
Therefore we switch the BB_C0012 to another LacI-coding sequence that excluded the degradation tail., called LacIB115 which is in psB1AK3. With the restriction enzymes ApaI and PstI , both restriction sites surround the LacI coding region for psB1AK3 and I-construct. Afterdigestion of both plamids, you run the gel and cut out the band with LacI (without the degradation tail and the I-construction backbone and assembly those two parts.
 "
"