|
JULY: WEEK 4
July, 19th
TECAN test showed that no RFP was produced from our parts, so all parts are potentially correct! For this reason we decided to sequence:
- I14-1 (Forward)
- I16-1 (Forward)
- I17-1 (Forward)
- I18-1 (Forward)
- I19-1 (Forward)
We also sequenced:
- I74C5-2 (Forward and Reverse)
- I84C5-2 (Forward and Reverse)
- I12-2 (Forward and Reverse)
These samples were prepared for sequencing (DNA was essicated) and sent to BMR genomics.
Trasformation of RING into:
- BW25141 (pir+)
- BW25142 (pir116)
- BW23474 (pir116)
- DH5alpha
- MG1655
Cultures were plated on:
- BW2514: Cm 34ug/ml
- BW25142: Cm 34ug/ml
- BW234741: Cm 34ug/ml
- DH5alpha: Cm 12,5ug/ml
- MG1655: Cm 12,5ug/ml
Inoculum of:
- I3-1
- I10-1
- I12-2
- I14-1
- I17-1
- <partinfo>BBa_J23110</partinfo>
in 5ml LB+Amp. Cultures were grown ON 37°C 220 rpm.
BW23473 arrived from Yale University on a paper disk. It was grown ON in 5ml L, at 37°C, 220 rpm.
July, 20th
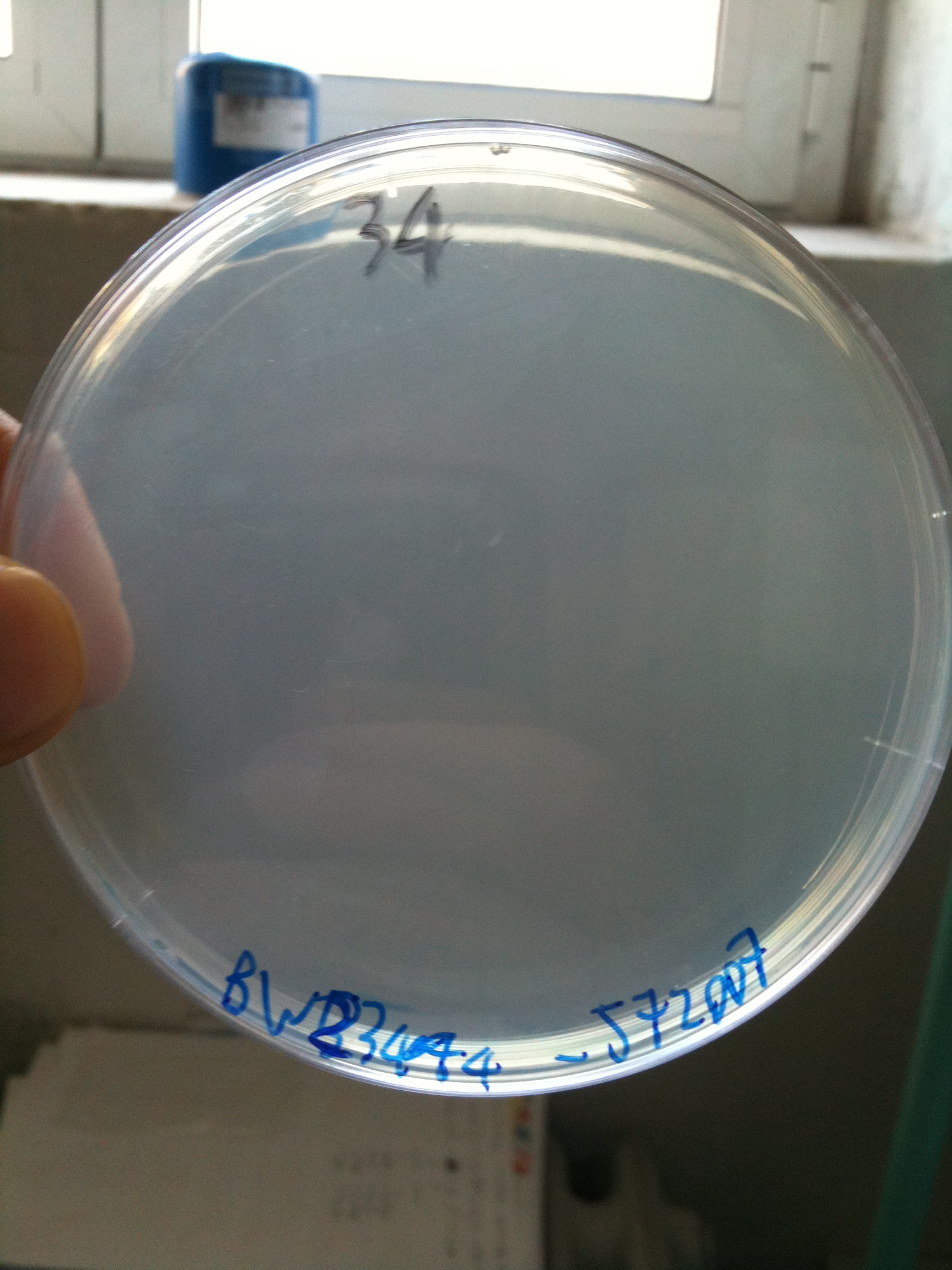 BW23474 transformed with <partinfo>BBa_J72007</partinfo> | 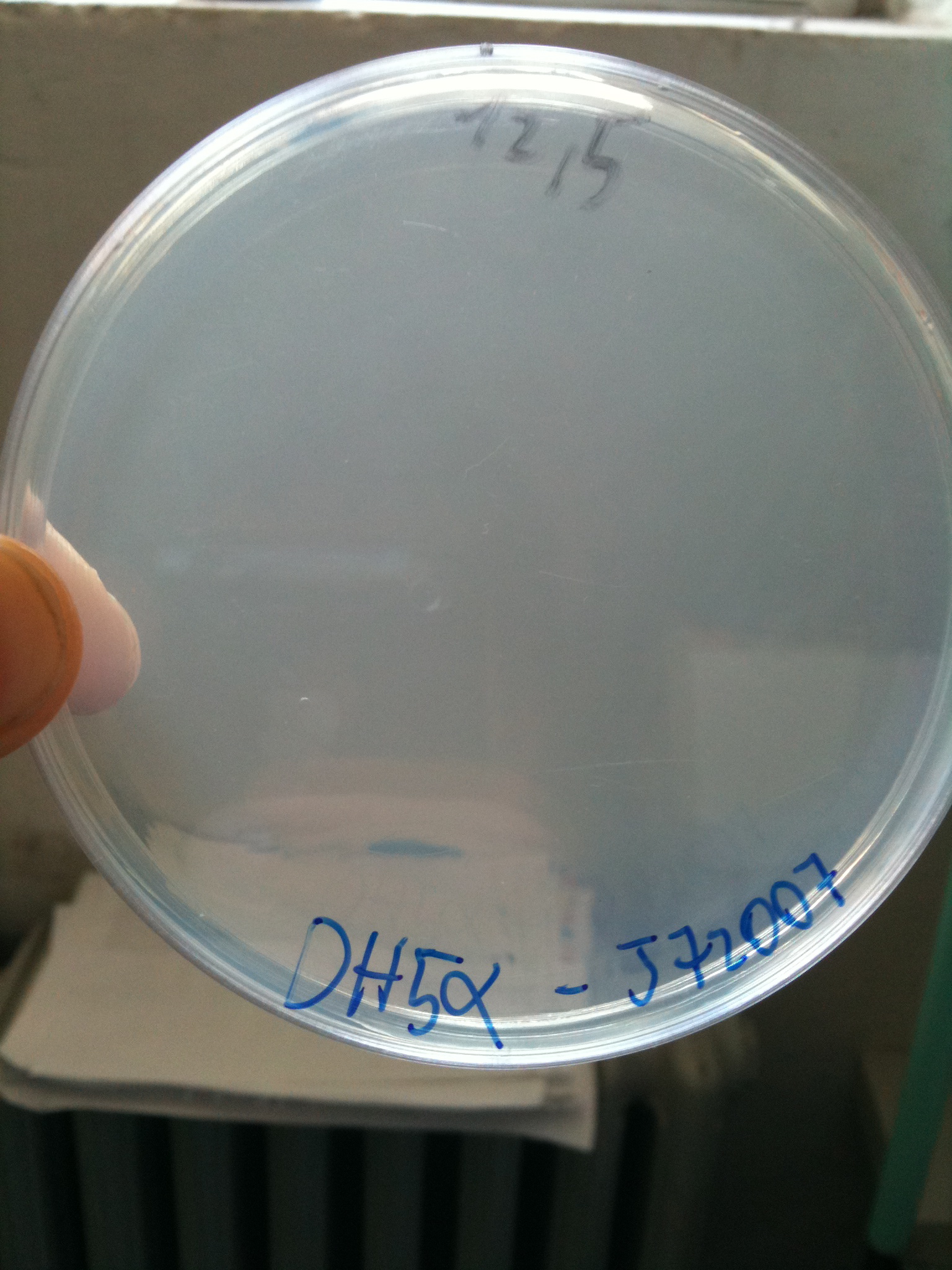 DH5alpha transformed with <partinfo>BBa_J72007</partinfo> |
Results for plates incubated ON, 37° C:
- BW23474 (pir116): showed colonies
- BW25141 (pir+): showed colonies
- BW25142 (pir116): showed colonies
- DH5alpha: didn't show colonies
- MG1655: didn't show colonies (even if there were a very few colonies that we suppose integrated the resistance of RING to survive - or the plate antibiotic wasn't homogeneous)
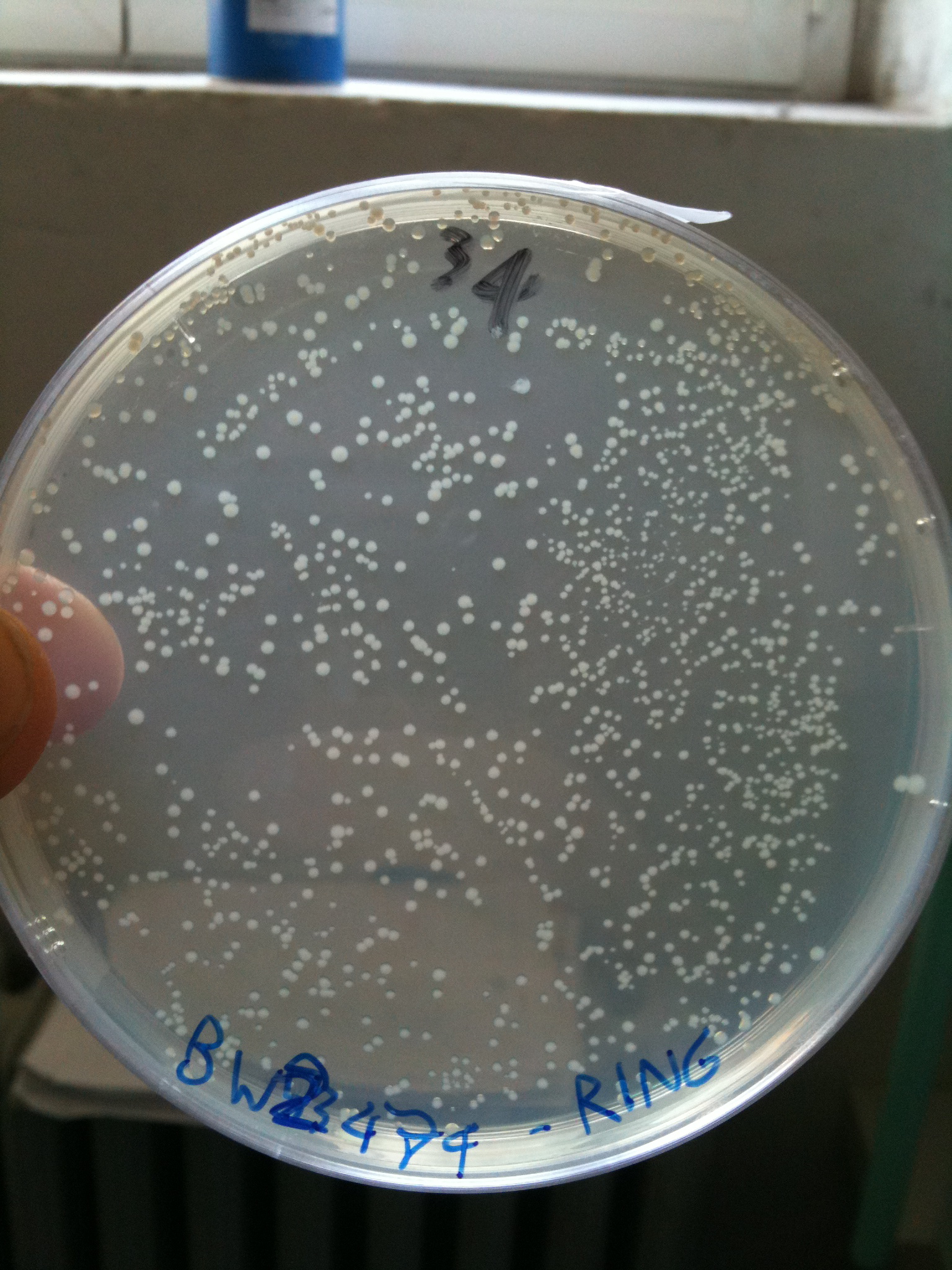 BW23474 transformed with RING | 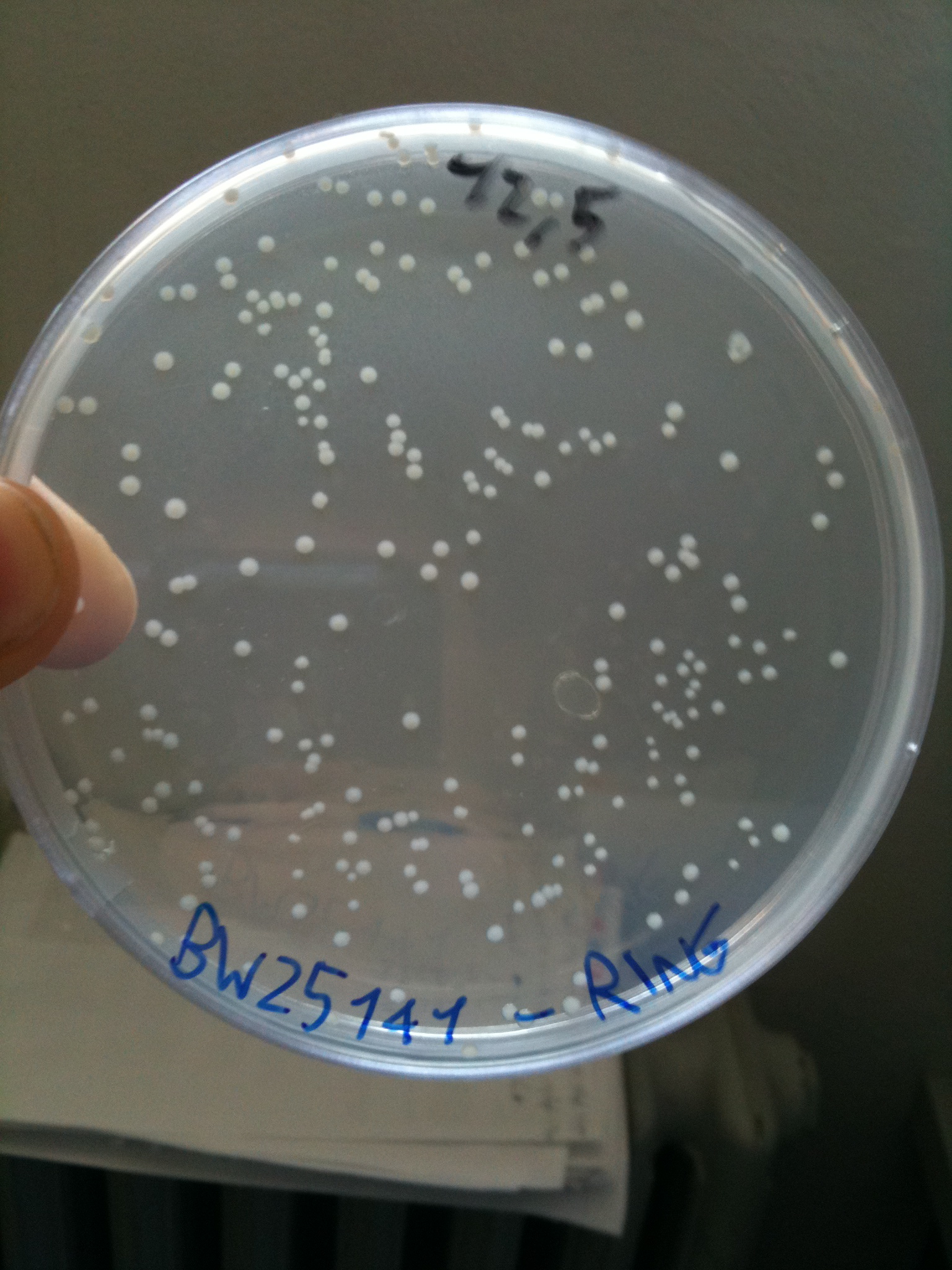 BW25141 transformed with RING | 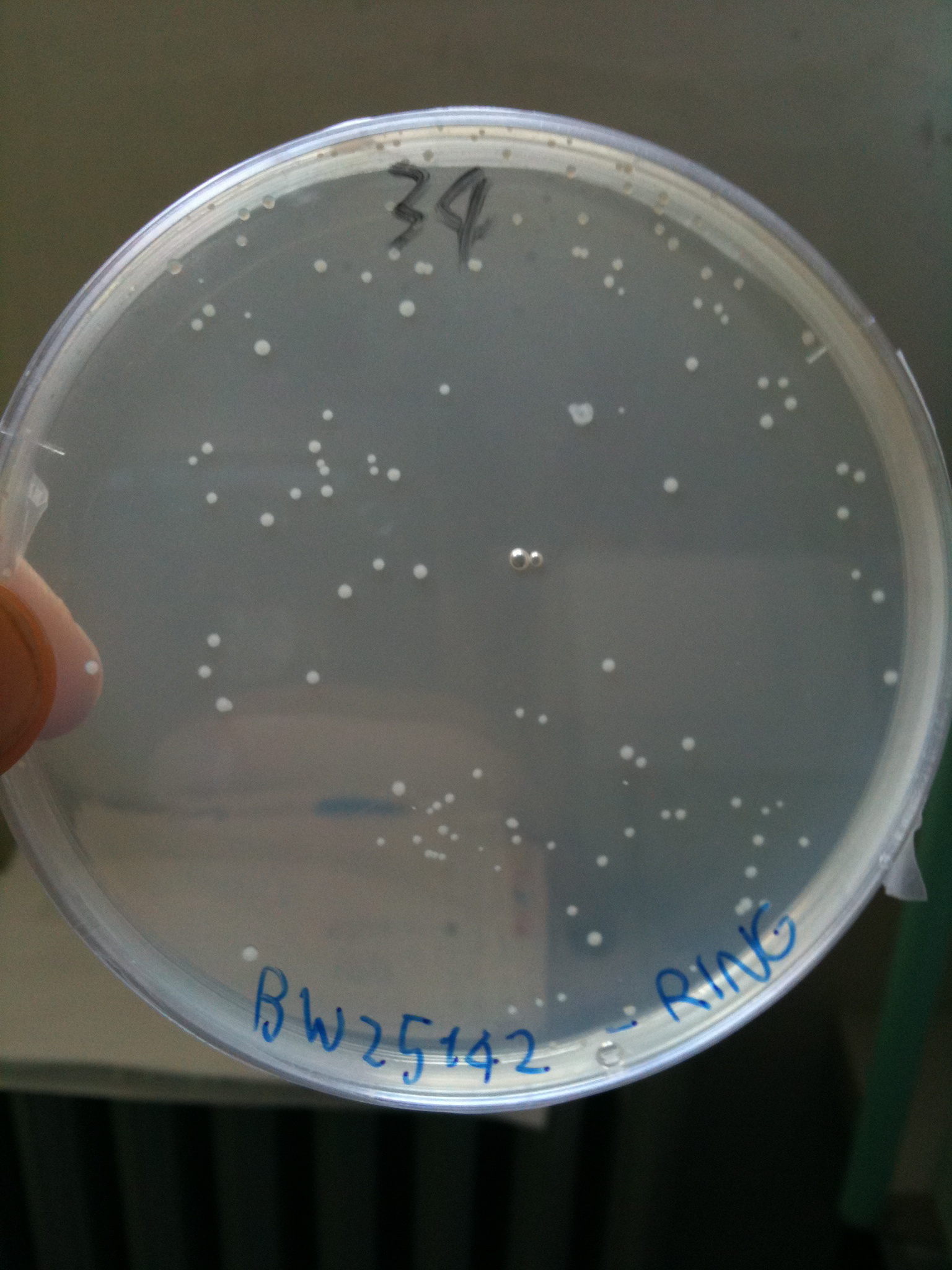 BW25142 transformed with RING |
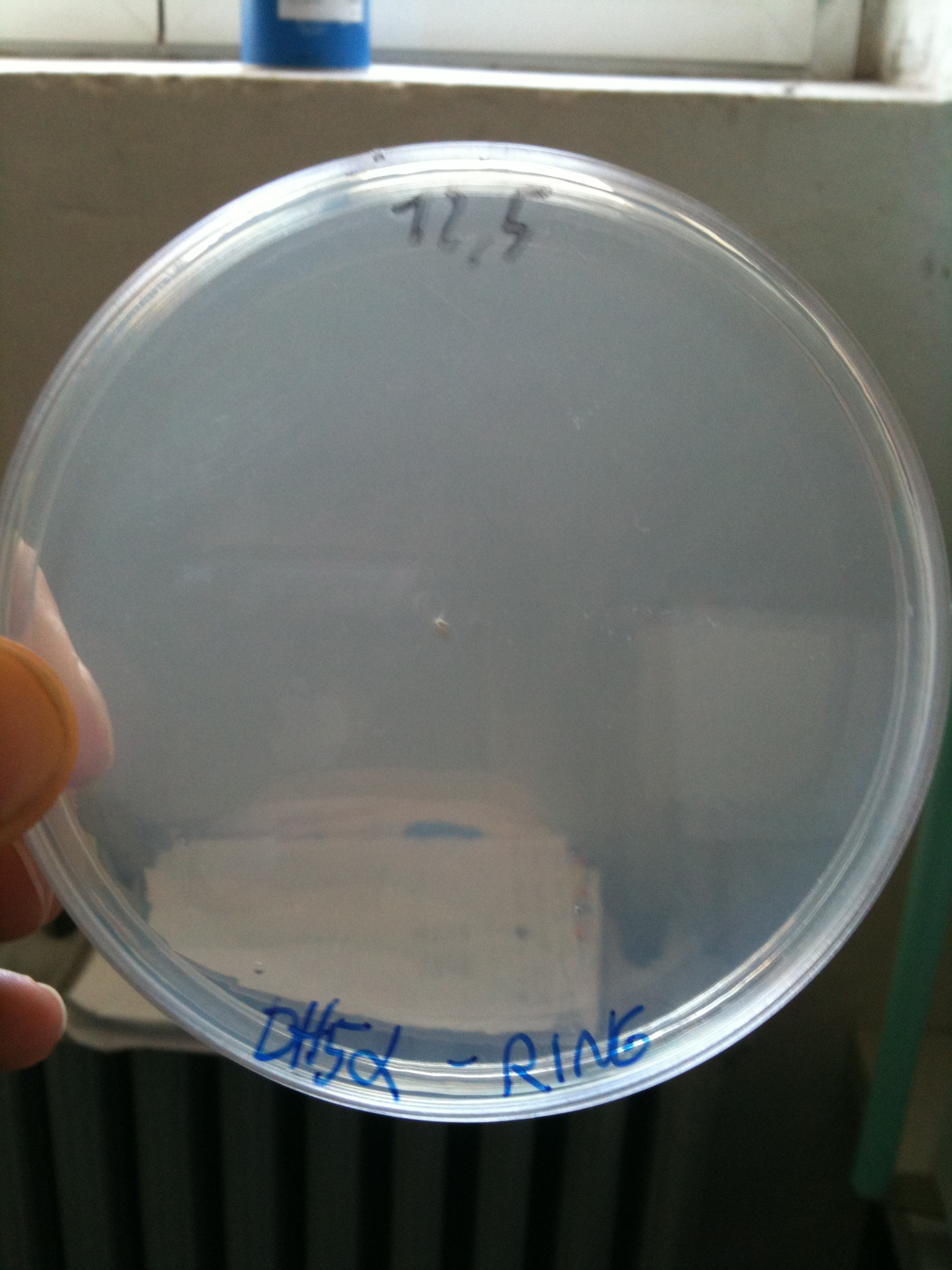 DH5alpha transformed with RING | 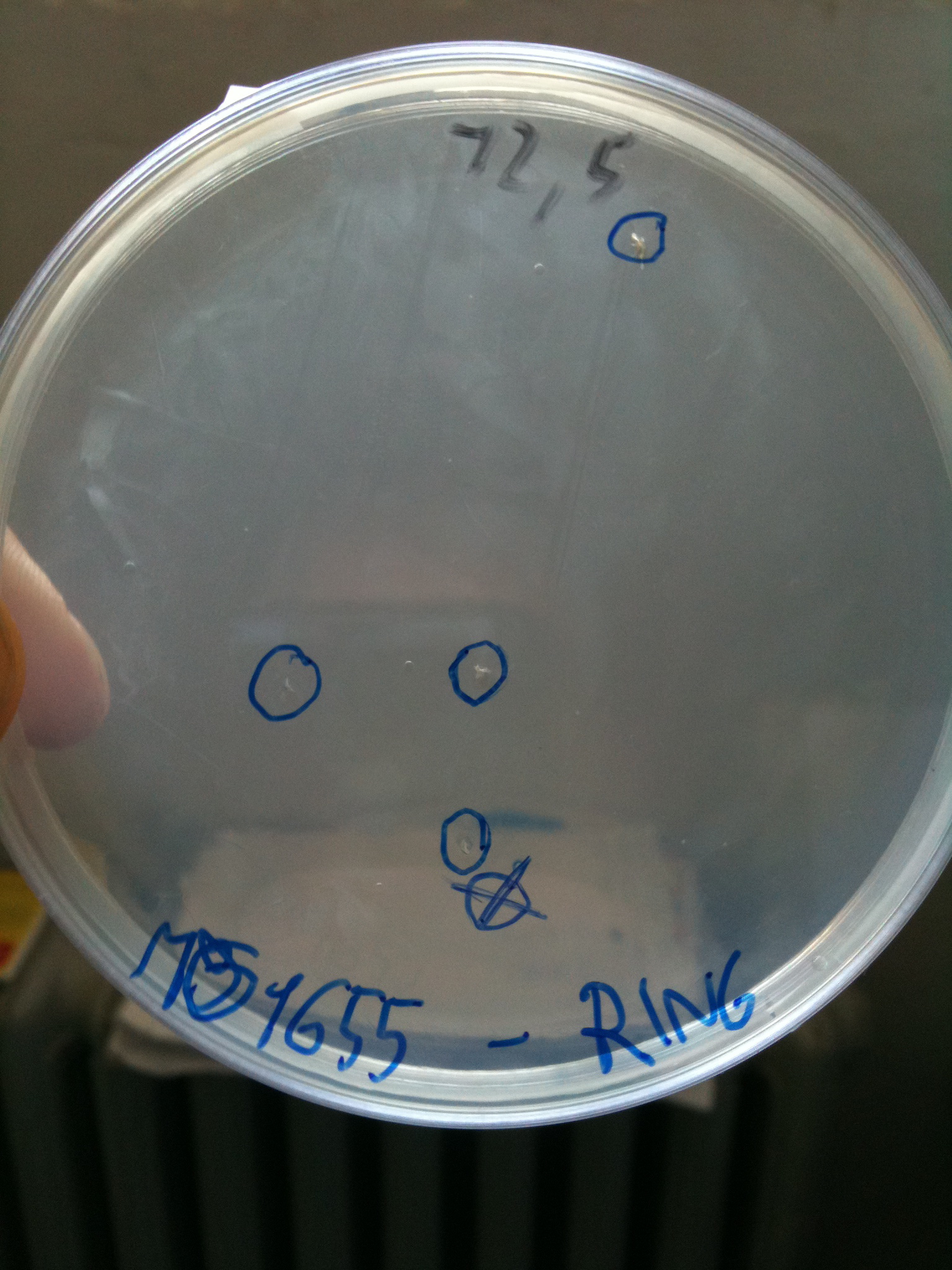 MG1655 transformed with RING |
Single colonies were picked from plates and grown in LB+Cm at proper concentration. MG1655 colonies were let grow in LB+Cm to check if they integrated the Cm resistance of RING.
MiniPrep was performed on cultures incubated yesterday, with following yields:
| Culture | Quantification
|
| I10-1 | 117.8 ng/ul
|
| I12-2 | 105,9 ng/ul
|
| I3-1 | 166,0 ng/ul
|
| I14-1 | 116,8 ng/ul
|
| I17-1 | 189,5 ng/ul
|
| <partinfo>BBa_J23110</partinfo> | 169,9 ng/ul
|
We retrieved from our freezer the following MiniPreps, with quantifications:
| Culture | Quantification
|
| 4C5 | 276 ng/ul
|
| I16-1 | 68,4 ng/ul
|
| I18-1 | 63,6 ng/ul
|
| I19-1 | 58,8 ng/ul
|
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer H
|
| <partinfo>BBa_J23110</partinfo> | Vector | 25 | 6 | 14,5 | 1 SpeI | 1 PstI | 2,5
|
| I3-1 | Vector | 25 | 10,5 | 10 | 1 XbaI | 1 PstI | 2,5
|
| 4C5 | Vector | 25 | 3,6 | 16,9 | 1 EcoRI | 1 PstI | 2,5
|
| I14-1 | Insert | 25 | 12,8 | 7,7 | 1 EcoRI | 1 PstI | 2,5
|
| I16-1 | Insert | 25 | 12,5 | 8 | 1 EcoRI | 1 PstI | 2,5
|
| I17-1 | Insert | 25 | 8 | 12,5 | 1 EcoRI | 1 PstI | 2,5
|
| I18-1 | Insert | 25 | 13 | 7,5 | 1 EcoRI | 1 PstI | 2,5
|
| I19-1 | Insert | 25 | 13 | 7,5 | 1 EcoRI | 1 PstI | 2,5
|
| I12-2 | Insert | 25 | 14 | 6,5 | 1 EcoRI | 1 PstI | 2,5
|
| I10-1 | Insert | 25 | 12,5 | 8 | 1 EcoRI | 1 PstI | 2,5
|
These parts were gel run/cut:
| I3-1 (X-P) | 5,9 ng/ul
|
| <partinfo>BBa_J23110</partinfo> (S-P) | 20,2 ng/ul
|
| I14-1 (E-P) | 13,0 ng/ul
|
| I16-1 (E-P) | 5,5 ng/ul
|
| I17-1 (E-P) | 11,9 ng/ul
|
| I18-1 (E-P) | 8,4 ng/ul
|
| I19-1 (E-P) | 4,4 ng/ul
|
4C5, I12-2 and I10-1 couldn't be extracted from gel, because bands were insignificant. For this reason we decided not to perform ligations involving I12-2 and I10-1 today. Luckily we retrieved from our freezer 4C5(E-P), so we could perform following ligations:
- I15new=<partinfo>BBa_J23110</partinfo> (S-P) + I3-1 (X-P)
- I14_4C5=I14(E-P)+4C5(E-P)
- I16_4C5=I16(E-P)+4C5(E-P)
- I17_4C5=I17(E-P)+4C5(E-P)
- I18_4C5=I18(E-P)+4C5(E-P)
- I19_4C5=I19(E-P)+4C5(E-P)
Ligations were incubated overnight at 16°C.
Glycerol stock for BW23473.
Our glycerol was contaminated, so we decided to prepare it again!
BW23474-RING was re-inoculated because it is still not grown.
We incoulated from glycerol stock (3ul in 2ml LB+Amp):
- <partinfo>BBa_J23110</partinfo>
- <partinfo>BBa_J23118</partinfo>
- <partinfo>BBa_J23116</partinfo>
- <partinfo>BBa_J23114</partinfo>
- <partinfo>BBa_J23106</partinfo>
- <partinfo>BBa_J23105</partinfo>
- <partinfo>BBa_J23101</partinfo>
- <partinfo>BBa_J23100</partinfo>
- <partinfo>BBa_B0033</partinfo>
in order to perform a TECAN test tomorrow.
Inoculum of MC1061 in LB, PBHR68 in LB+Amp and <partinfo>BBa_T9002</partinfo> in LB+Amp to prepare competent cells tomorrow.
July, 21st
This morning all cultures were grown:
- BW25141-RING
- BW25142-RING
- BW23474-RING
- BW23474-RING re.inoculated yesterday night (thrown away because unuseful)
- MG1655-1
- MG1655-2
- MG1655-3
- MG1655-4
For these 7 seven cultures glycerol stocks were prepared and stored at -80°C. Remaning 5 ml were used to perform MiniPrep.
After MiniPrep, purified DNA was quantified with NanoDrop.
| Culture | Quantification
|
| BW25141-RING | 20,9 ng/ul
|
| BW25142-RING | 31,8 ng/ul
|
| BW23474-RING | 36,5 ng/ul
|
| MG1655-1 | 3,5 ng/ul
|
| MG1655-2 | 24 ng/ul
|
| MG1655-3 | 10,6 ng/ul
|
| MG1655-4 | 25,7 ng/ul
|
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer B
|
| MG1655-1 | Screening | 25 | 20 | 0,5 | 1 HindIII | 1 HindIII | 2,5
|
| MG1655-2 | Screening | 25 | 10 | 10,5 | 1 HindIII | 1 HindIII | 2,5
|
| MG1655-3 | Screening | 25 | 20 | 0,5 | 1 HindIII | 1 HindIII | 2,5
|
| MG1655-4 | Screening | 25 | 10 | 10,5 | 1 HindIII | 1 HindIII | 2,5
|
| BW25141-RING | Screening | 25 | 10 | 10,5 | 1 HindIII | 1 HindIII | 2,5
|
| BW25142-RING | Screening | 25 | 8 | 12,5 | 1 HindIII | 1 HindIII | 2,5
|
| BW23474-RING | Screening | 25 | 8 | 12,5 | 1 HindIII | 1 HindIII | 2,5
|
<partinfo>BBa_J23116</partinfo>
positive control | Screening | 25 | 2 | 18,5 | 1 HindIII | 1 HindIII | 2,5
|
Today we also prepared competent cells for cultures inoculated yesterday:
- <partinfo>BBa_T9002</partinfo> (already resistent to Ampicillin, grown in LB+Amp)
- MC1061 (grown in LB with no antibiotic)
- PBHR68 (already resistent to Ampicillin, grown in LB+Amp)
In the afternoon, we transformed our ligations:
Trasformation of ligations in:
| Ligation name | E. coli strain | Resistance |
|---|
- I15new=<partinfo>BBa_J23110</partinfo> (S-P) + I3-1 (X-P)
|
DH5alpha
|
Amp 100 |
- I14_4C5=I14(E-P)+4C5(E-P)
|
DH5alpha
|
Amp 100+Cm 12,5 |
- I16_4C5=I16(E-P)+4C5(E-P)
|
DH5alpha
|
Amp 100 + Cm 12,5 |
- I17_4C5=I17(E-P)+4C5(E-P)
|
DH5alpha
|
Amp 100 + Cm 12,5 |
- I18_4C5=I18(E-P)+4C5(E-P)
|
DH5alpha
|
Amp 100 + Cm 12,5 |
- I19_4C5=I19(E-P)+4C5(E-P)
|
DH5alpha
|
Amp 100 + Cm 12,5 |
Plates were incubated overnight at 37°C.
July, 22nd
Plates results were not nice: I15 new showed few colonies (about 20 colonies), I14_4C5 showed 2 colonies, I16_4C5 showed 3 colonies and I18_4C5 1 colony, while for other plates no colony was observed, probably due to the fact that our 4C5 (E-P) plasmid retrieved from freezer was too old and not usable anymore. For this reason, we decided to perform a colony PCR on available colonies of I14_4C5, I16_4C5 and I18-4C5, on 3 colonies of I15new and to repeat wrong ligations and the one including I10-1 and I12-2 (their vector had to be changed with pSB4C5).
Colonies were saved in 1ml LB+suitable antibiotic/s. After gel results positive colonies will be glycerol stocked.
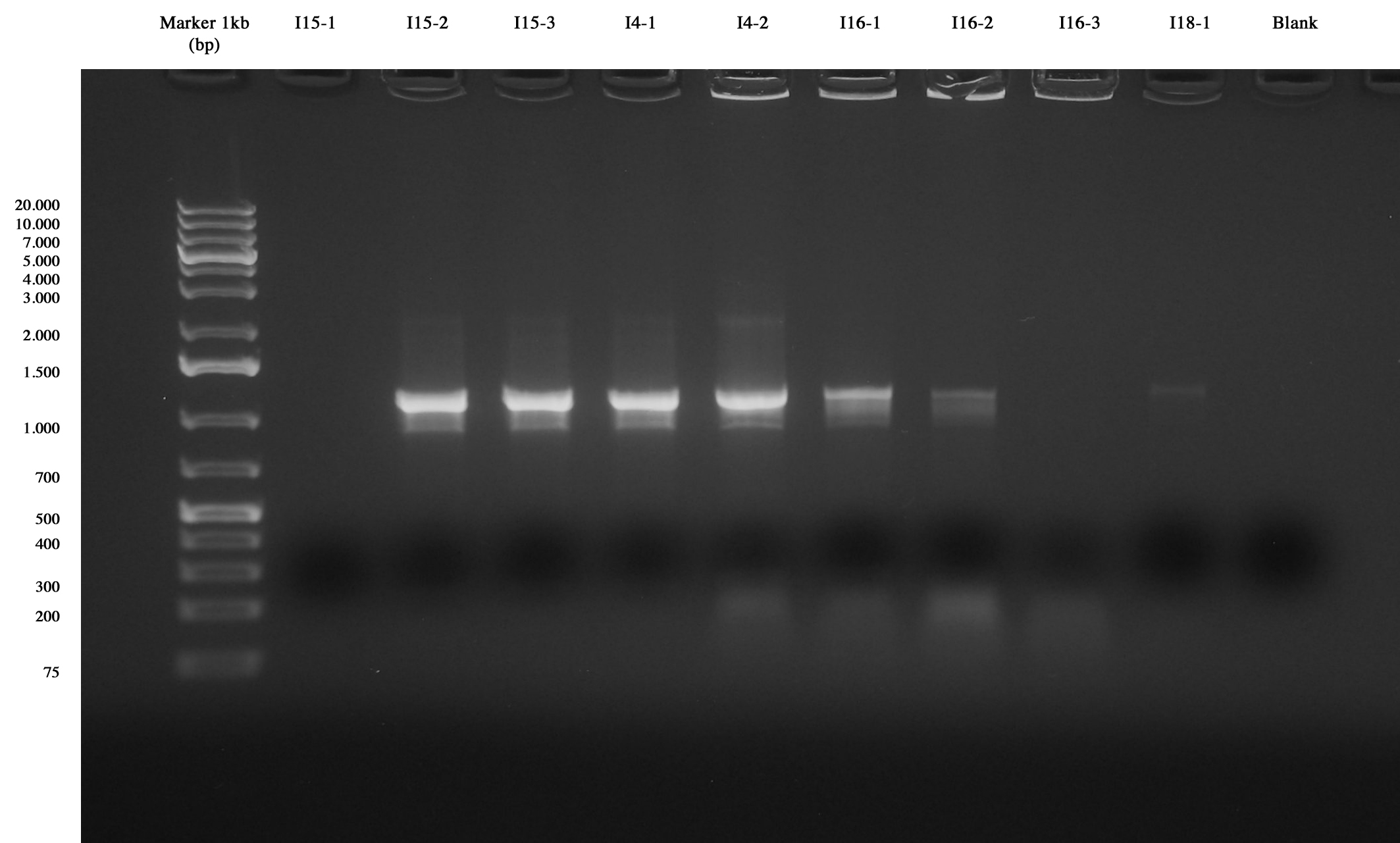 Colony PCR of colonies picked from plates Colony PCR and gel run showed that I15-1, I15-2 and I15-3 have the right insert. Also for I14_4C5-1 and I14_4C5-2 the insert was visible. For these parts we decided to perform a NheI-PstI screening. I16-4C5-1 and I16_4C5-3 showed the insert, so they will be screened as before. No band was observed for I16_4C5-2 and I18_4C5-1 (This ligation will be repeated).
Glycerol stok was prepared for I14_4C5-1, I14_4C5-2, I16_4C5-1, I16_4C5-3, I15new-1, I15new-2 and I15new-3. Falcon tubes containing the remaining culture were re-filled with 5ml LB+antibiotic and tomorrow will be screened.
Other parts (I14 E-P, I16 E-P, I17 E-P, I18E-P, I19 E-P) were available in the freezer, so only 3 parts still had to be digested.
Digestion of pSB4C5 (E-P), I12-2(E-P) and I10-1(E-P) was repeated as yesterday, digestions were gel run/cut and bands were visible, so this time gel extraction was performed with the following quantifications:
- pSB4C5 (E-P): 23,0 ng/ul
- I10-1 (E-P): 14,1 ng/ul
- I12-2 (E-P): 21,9 ng/ul
Following ligations were performed:
- I17_4C5=I17(E-P)+4C5(E-P)
- I18_4C5=I18(E-P)+4C5(E-P)
- I19_4C5=I19(E-P)+4C5(E-P)
- I10-4C5=I10(E-P)+4C5(E-P)
- I12-4C5=I12(E-P)+4C5(E-P)
In the afternoon, competent T9002 (on LB+Amp+Cm 12,5 LB agar plates) and MC1061 (on LB+Cm 12,5 agar plates) cells were transformed with a negative control (ENTERO 4C5) in pSB4C5, in order to evaluate transformation efficiency.
Plates were incubated at 37°C overnight.
We also performed a TECAN test in order to evaluate the ranking of sternght of promoters we inoculaed yesterday. Results how that the ranking is the one reported here (from stronger to weaker):
- <partinfo>BBa_J23100</partinfo>
- <partinfo>BBa_J23101</partinfo>
- <partinfo>BBa_J23110</partinfo>
- <partinfo>BBa_J23118</partinfo>
- <partinfo>BBa_J23106</partinfo>
- <partinfo>BBa_J23105</partinfo>
- <partinfo>BBa_J23116</partinfo>
- <partinfo>BBa_J23114</partinfo>
Today we also prepared new plates, because we finished the one prepared, for our intensive lab activity...
50 plates LB+Amp+Cm 12,5.
We transformed pSB4C5 (4ng) in our home made competent cells:
to evaluate transformation efficiency.
July, 23rd
All plates incubated yesterday (T9002-4C5, MC1051-4C5 and PBHR68-4C5) showed colonies! We decided to incubate T9002 for furthrt time, because colonies were too small, before counting... Soon we will count colonies to evaluate efficiency of transformation for our home made competent cells!
Today we transformed our ligations:
| Ligation name | E. coli strain | Resistance |
|---|
- I17_4C5= I17 (E-P) + pSB4C5 (E-P)
|
DH5alpha
|
Cm 12,5 |
- I18_4C5= I18 (E-P) + pSB4C5 (E-P)
|
DH5alpha
|
Cm 12,5 |
- I19_4C5= I19 (E-P) + pSB4C5 (E-P)
|
DH5alpha
|
Cm 12,5 |
- I10_4C5= I0 (E-P) + pSB4C5 (E-P)
|
TOP10
|
Cm 12,5 |
- I12_4C5= I12 (E-P) + pSB4C5 (E-P)
|
TOP10
|
Cm 12,5 |
|
|
PBHR68
|
Amp 100+Cm 12,5 |
|
|
MC1061
|
Cm 12,5 |
- No DNA (negative control)
|
MC1061
|
Cm 12,5 |
After MiniPrep, purified DNA was quantified with NanoDrop.
| Culture | Quantification
|
| I15-1 | 34,2 ng/ul
|
| I15-2 | 57,1 ng/ul
|
| I15-3 | 72 ng/ul
|
| I14_4C5-1 | 14,7 ng/ul
|
| I14_4C5-2 | 20,9 ng/ul
|
| I16_4C5-1 | 16,3 ng/ul
|
| I16_4C5-2 | 15,3 ng/ul
|
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer B
|
| I15-1 | Screening | 25 | 3 | 18,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I15-2 | Screening | 25 | 3 | 18,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I15-3 | Screening | 25 | 3 | 18,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I14_4C5-1 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I14_4C5-2 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I16_4C5-1 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I16_4C5-2 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
Digestions were incubated for 3h at 37°C, then gel run.
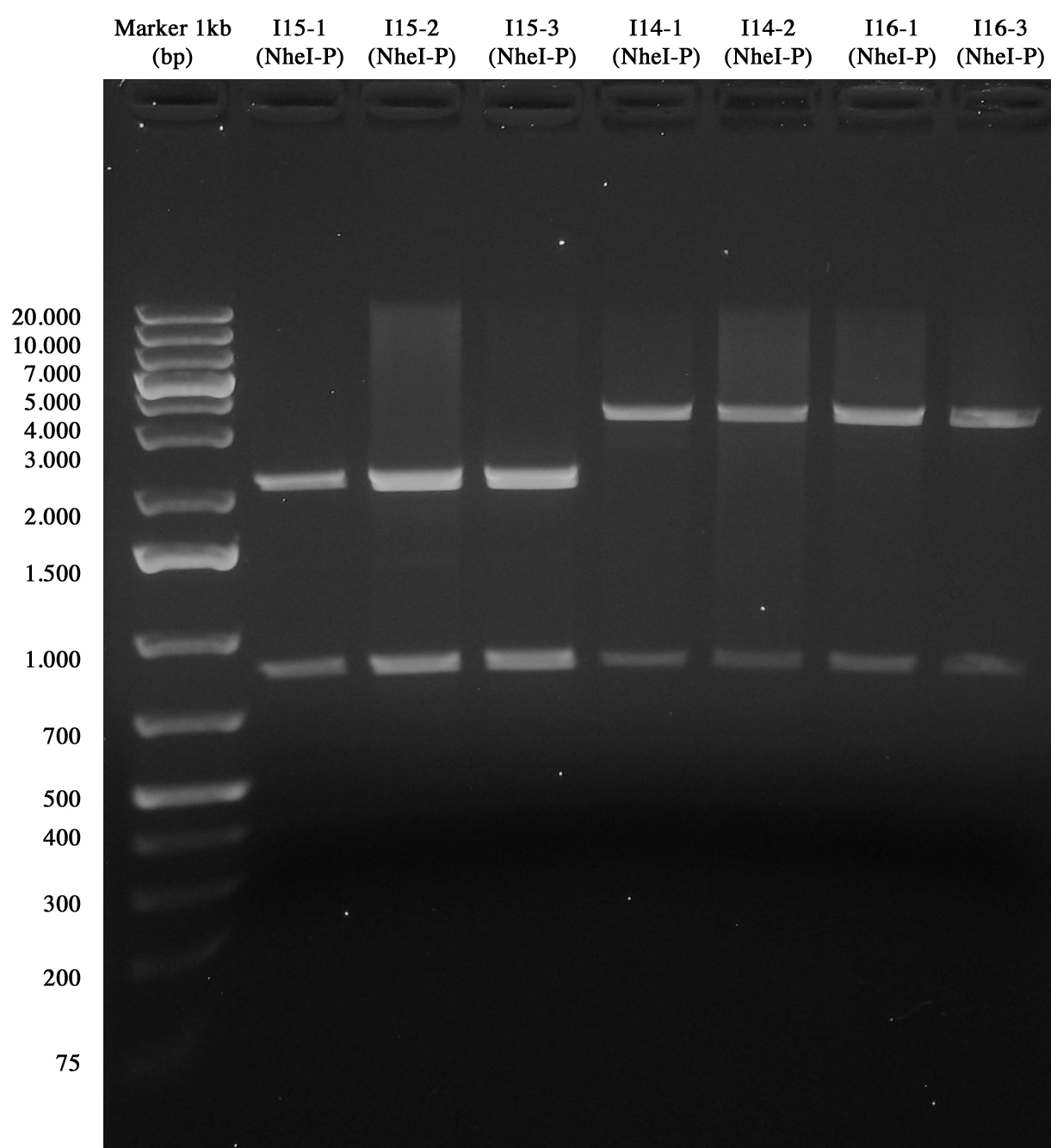 Screening for I15, I14-4C5 and I16_4C5 Gel showed that all parts were ok!!
We also received sequencing results:
|
I14-1 |
correct! |
|
I16-1 |
correct! |
|
I17-1 |
correct! |
|
I18-1 |
correct! |
|
I19-1 |
correct! |
|
I12-2 |
correct! |
|
I7_4C5-2 |
correct! |
|
I8_4C5-2 |
correct! |
Our plate with MC1061 transformed with RING showed few contamination, so we decided to repeat the transformation.
July, 24th
We retransformed RING in MC1061 in order to see if contamination we noticed yesterday was due to tranformation or not. Plate was incubated overnight at 37°C and tomorrow we will evaluate efficiency.
For plates incubated yesterday, we picked 2 colonies for each plate:
- I10_4C5-1
- I10_4C5-2
- I12_4C5-1
- I12_4C5-2
- I17_4C5-1
- I17_4C5-2
- I18_4C5-1
- I18_4C5-2
- I19_4C5-1
- I19_4C5-2
Efficiency of transformation:
| Culture | Colonies
|
| BW25141 | 232 colonies
|
| BW25142 | 137 colonies
|
| BW23474 | 1436 colonies
|
July, 25th
The plate containing RING in MC1061 showed 3 colonies! These colonies were screened and grown in LB+Cm 12,5. For colonies picked yesterday screening digestion was performed.
MiniPrep was performed for:
- I10_4C5-1
- I10_4C5-1
- I12_4C5-1
- I12_4C5-1
- I17_4C5-1
- I17_4C5-1
- I18_4C5-1
- I18_4C5-1
- I19_4C5-1
- I19_4C5-1
Digestion of:
| Culture | Kind | Final reaction volume (ul) | DNA (ul) | H20 (ul) | Enzyme 1 | Enzyme 2 | Buffer Tango
|
| I10-4C5-1 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I10-4C5-1 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I12-4C5-1 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I12_4C5-2 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I17_4C5-1 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I17_4C5-2 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I18_4C5-1 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I18_4C5-2 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I19_4C5-1 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
| I19_4C5-2 | Screening | 25 | 10 | 11,5 | 0,5 NheI | 0,5 PstI | 2,5
|
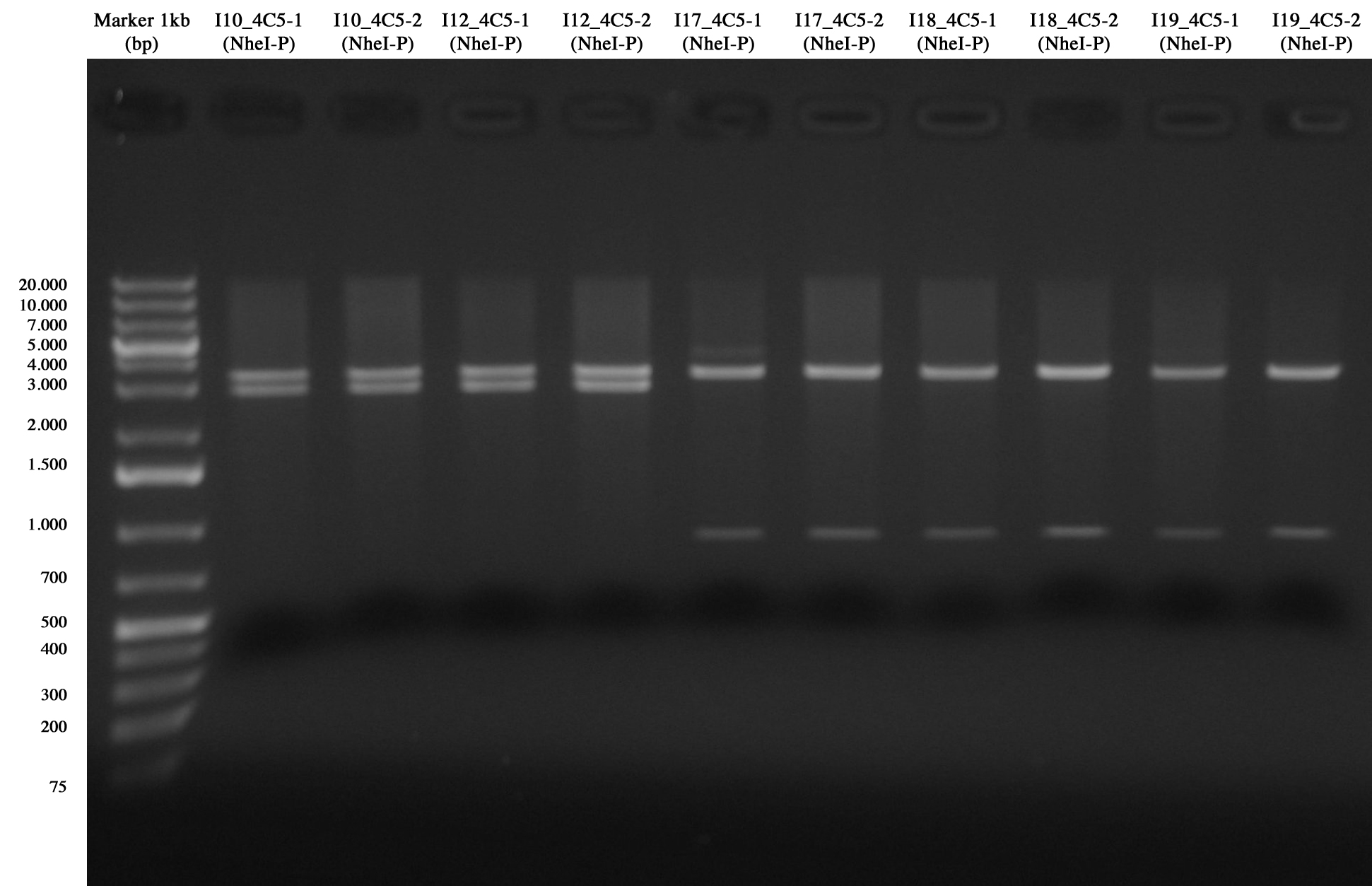 Screening for I10_4C5, I12_4C5, I17_4C5, I18_4C5 and I19_4C5
From gel it is possible to see that all of them are positive!! :)
|
|
 "
"










