Team:TU Munich/Project
From 2010.igem.org
|
||||||||||||||||
|
|
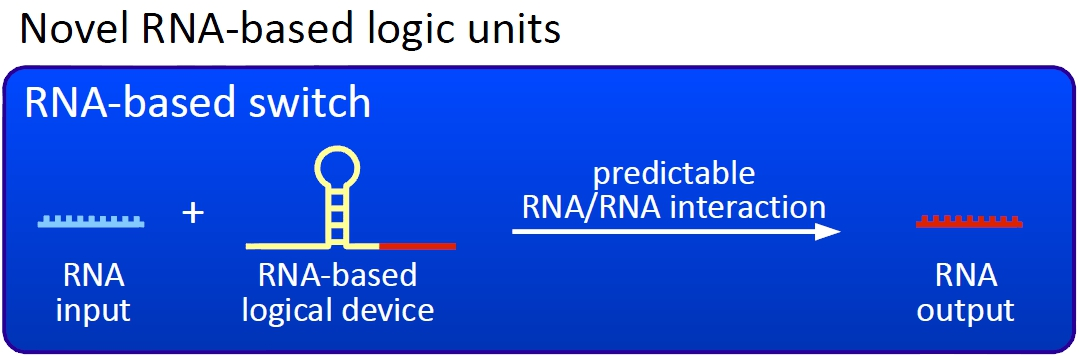
AbstractOriginally, the idea of iGEM was to create and use biological parts to achieve novel features in biological systems. However, most of the participants concentrate on the first with the latter falling behind. This is what we want to change with our project. By developing a biological network based on logical devices we want to offer everyone the opportunity to 'program' their own cells with individual AND/OR/XOR connections between BioBricks of their choice. Thereby, BioBricks can finally fulfill their original assignment as biological parts which can be connected in many different ways. We want to achieve this by engineering simple and easy-to-handle switches based on predictable RNA/RNA-interactions. Our projectOur visionWe, the TU Munich 2010 team, would like to change the usage and handling of Biobricks. Over the years many teams participating in the iGEM competition spent their time on evolving and constructing receptors and systems to detect a certain input that a variety of gorgeous oppurtunities is available so far. Nevertheless, until now it is not possible to link all those functionalities and build up a network giving differenciated responses to several of those input signals, where the molecular response depends on the complex composition of the environment a cell faces. We would like to offer this possibility to everyone.
The conceptThe concept we rely on for our design of RNA-switches is based on the principle of attenuation. We applied this principle of RNA-RNA interaction based regulation to build switches based on antitermination. Note, that our systems work with termination on transcription level, so in our case the RNA-Polymerase falls off instead of stalling a ribosome. Antitermination means the avoidance of trancription termination by interaction with another RNA sequence (the signal), which binds to the transcription mRNA and anticipates the formation of a stem loop. So basicly one gets a yes/no answer with a first switch: If the small RNA piece is available, transcription will continue. If not, it will be terminated. This is the way our first devices should work. Now, what's the great thing about our system? Well, in principle we can construct a lot of those switches. The only things you need are complementary RNA sequences and one of them must form a stem loop big enough to terminate transcription (switch) if the other one (signal) is not there. Those RNA-RNA-interactions can easily be calculated and RNA structures can be predicted with high accuracy - in complete opposite to anything based on proteins. So we are easily capable of scaling up our switch and add a couple more of those guys into a bacteria cell (they are really small, both switch and signal RNA). Since we can use RNA switches to regulate RNA output, we can easily built up networks, with our RNA pieces as the major controlling element. In comparison to proteins, which are normally used for work like this, RNA is predictable in its structure, fast in production and fast in degeneration, so quick and time-related responses are possible, non-toxic in all cases, really cute, available in all shapes, consists only of a few sugars, phosphates, and four bases, there is an nearly endless pool of possible switches... You need more? Well, one more we would really like to mention: RNA can be generated using DNA. Big news? It was in the Watson/Crick era! Well, since RNA can be generated using DNA, you can really really easily code for our RNA switches using certain DNA sequences. DNA is stable and quite easy to get into cells and wait, Biobricks consists of DNA! So this is how we would like to revolutionize Biobricks. We developed switches based on a very easy, yet totally new principle, which can be just cloned in between known Biobricks and offer totally new possibilities in combining bricks and gene regulation! Check here for more information, a cute Java applet to form your own network and enjoy! The ExperimentsFluorescent proteins as reporterOur initial idea to prove our concept of antitermination was to use flourescent proteins as reporters. This approach gives the opportunity to measure the termination and antitermination efficiency of our designed BioBricks in vivo as well as in vitro, the latter using a translation kit based on e.coli lysate.
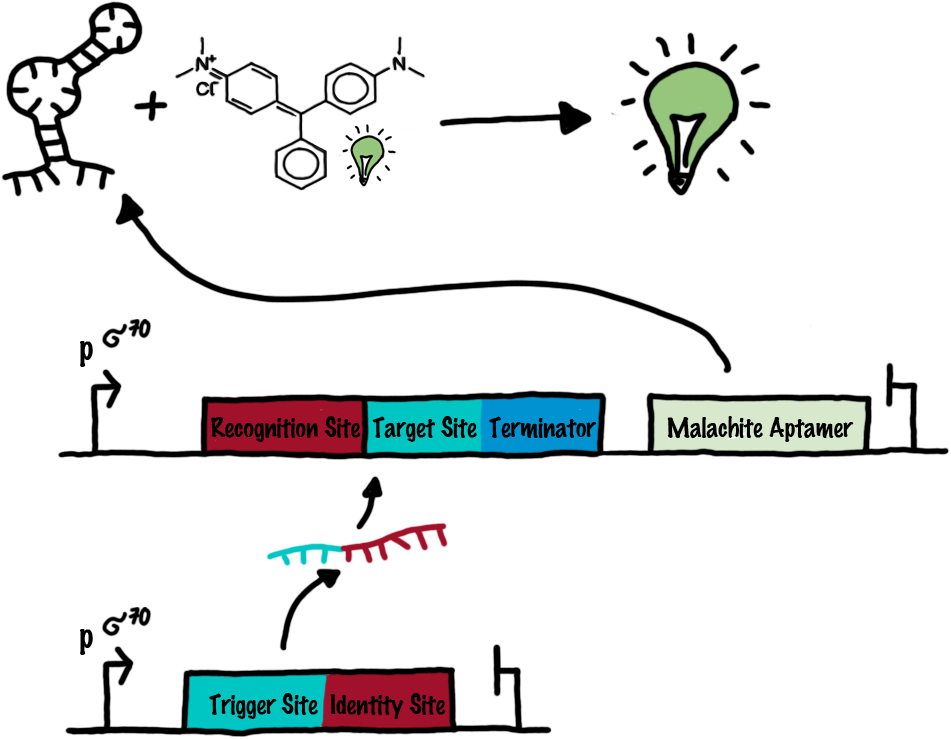
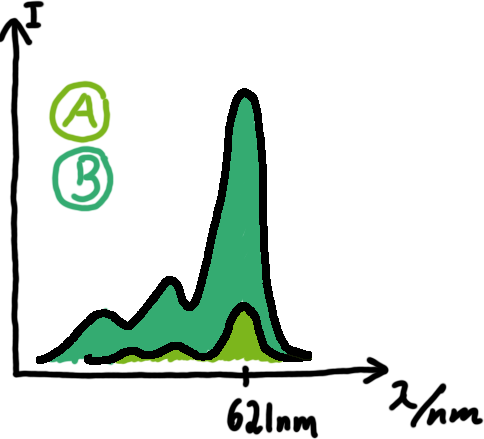
Wiith these measurements, it should also be possible to observe differences in efficiency of termination as well as antitermination between our designed switches. Measurements with the malachite green aptamer as reporterA second possibility to measure parameters of our switches we came up with, was the idea to investigate our system on the transcriptional level only. Therefore, we decided to use malachite green as reporter. Malachite green in a fluorescent dye, whose emission increasing dramaticly (about 3000 times) upon binding of a specific RNA-aptamer. ---concept to be desribed, as well as literature---
<ref>refs</ref>
Also we made constructs, where the transcription of the signal-RNA is under the control of a sigma(70) promoter. These two linear DNA-constructs, together with the e.coli RNA-polymerase and the right buffer conditions should represent an easy-to-handle measurement kit on the transcriptional level.
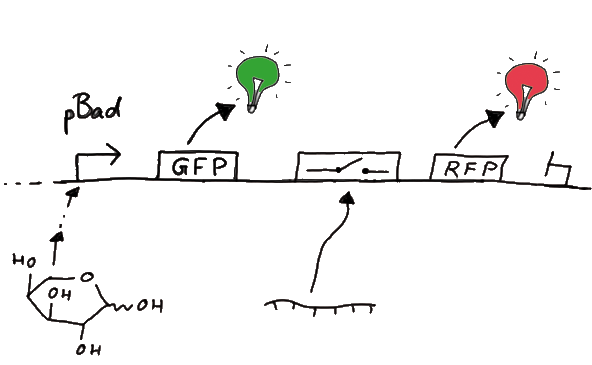
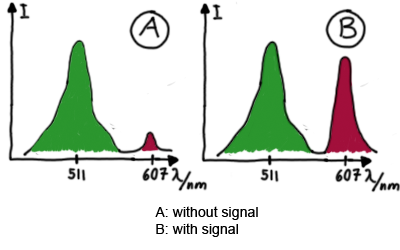
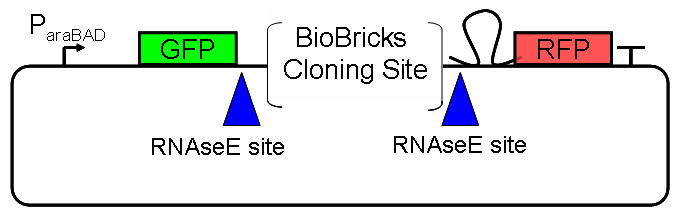
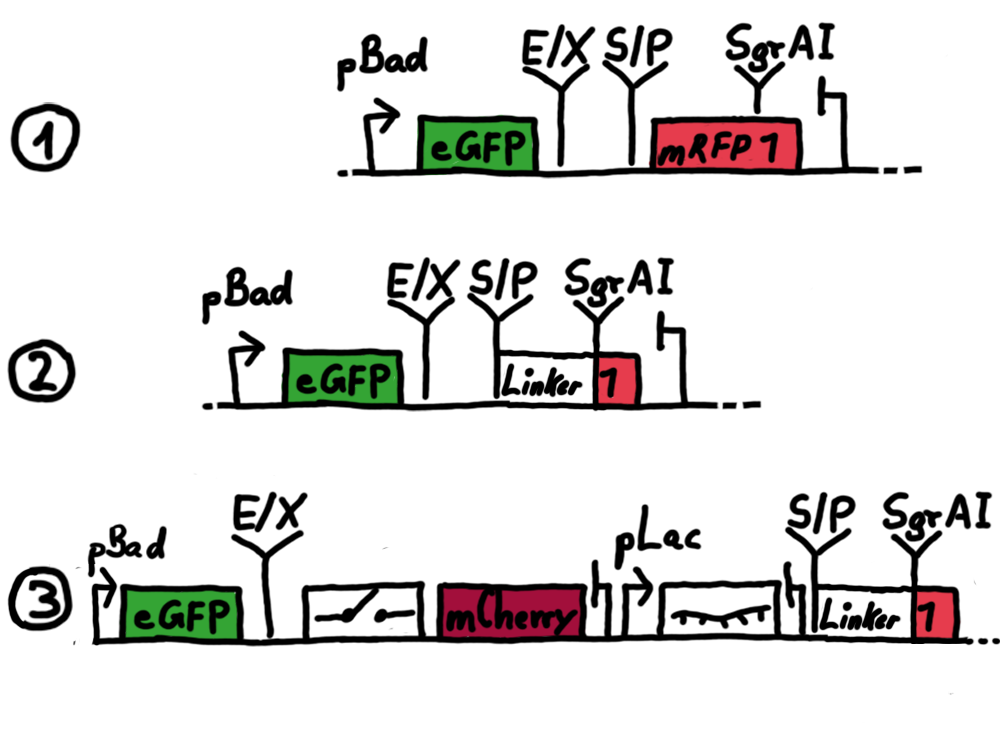
ResultsFlourescent proteinsUnfortunatly, we had to change the reporter construct two times during our experiments as several problems occured in our measurements: First Try: based on the measurement plasmid pSB1A10At the beginning, we decided to use the reporter plasmid [http://partsregistry.org/Part:pSB1A10 pSB1A10] from the registry. It consists of the fluorescent proteins eGFP and mRFP1. Each sequence includes a ribosome binding site and a stop-codon; the two genes are divided by a cloning side including the BioBrick cleavage sites.In front of the eGFP sequence, the plasmid includes an arabinose-inducable promoter. The plasmid also contains an ampicilline resistence. We cloned our switches into the cloning site of the measurement plasmid and used an empty cloning site as control; our signal-RNAs we cloned into the [http://partsregistry.org/Part:pSB1K3 pSB1K3] vector, together with the BioBricks R0011 (Lac promoter) and B0014 (double terminator of transcription). Afterwards, we cut pSB1K3 with Aat2 and Pst1 and pSB1A10 with Nsi1 and Aat2 and ligated those fragments of each plasmid that contained our Bricks to get a Monsterplasmid. We transformed BL21(DE3) cells with the plasmid. We set up cultures, induced the arabinose promoter and measured the GFP and mRFP1 excitation/emission spectra within time.
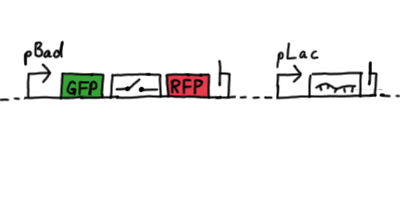
Second Try: A measurement plasmid of our own designTo design our own plasmid to overcome the problems that occurred in our first try gave us tghe possibility to overcome several other problems: Third Try: One promoter for each proteinWe decided to use the measuremnt plasmid we developed in our second try but to clone another L-arabinose induced promoter into the plasmid, in front of our switch followed by the mCherry sequence. In this way, we still can use GFP fluorescence as internal control, because both protein transcription is under the control of a promoter of identical design. Though we are still not able to tell exactly why our previous measurements did not work, but with this construct we measured the first time fluorescence of the mCherry protein in our positive control.
Discussionblablabla References<references/>
This is a template page. READ THESE INSTRUCTIONS.
You are provided with this team page template with which to start the iGEM season. You may choose to personalize it to fit your team but keep the same "look." Or you may choose to take your team wiki to a different level and design your own wiki. You can find some examples HERE.
You MUST have a team description page, a project abstract, a complete project description, a lab notebook, and a safety page. PLEASE keep all of your pages within your teams namespace.
|
|||||||||||||||
 "
"