Team:TU Munich/Lab
From 2010.igem.org
| Line 7: | Line 7: | ||
==In vivo Measurements== | ==In vivo Measurements== | ||
{{:Team:TU Munich/Templates/ToggleBoxStart}} | {{:Team:TU Munich/Templates/ToggleBoxStart}} | ||
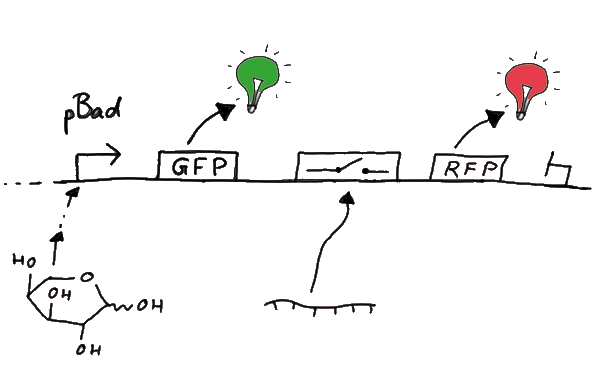
| - | + | Our initial idea to prove our concept of antitermination was to use flourescent proteins as reporters. This approach gives the opportunity to measure the termination and antitermination efficiency of our designed BioBricks ''in vivo'' as well as ''in vitro'', the latter using a translation kit based on <i>E. coli</i> lysate. <br>We decided to use the flourescent proteins GFP and RFP, as their spectra do not overlap and we would not measure any FRET. We would use GFP fluorescence as internal control and RFP fluorescence as signal to detect termination/antitermination by our switch we cloned in between the coding sequences of the proteins. Both protein sequences are under the control of one (L-arabinose induced) promoter. | |
| + | [[Image:TUM2010_gfprfp_schalter_klein.gif|center|our idea]] | ||
| + | <br> | ||
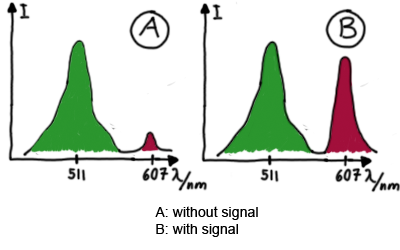
| + | When measuring the termination of our BioBricks and the antitermination by their corresponding signal-RNA, we should be able to observe an increasing RFP emission compared to the GFP emission upon induced signal-RNA production in the cells/in the kit:<br> | ||
| + | [[Image:TUM2010_Expected_emission_spextra.png|center|our idea]] | ||
| + | Wiith these measurements, it should also be possible to observe differences in efficiency of termination as well as antitermination between our designed switches. <br><br> | ||
{{:Team:TU Munich/Templates/ToggleBoxEnd}} | {{:Team:TU Munich/Templates/ToggleBoxEnd}} | ||
| + | |||
==In vitro Measurements== | ==In vitro Measurements== | ||
{{:Team:TU Munich/Templates/ToggleBoxStart}} | {{:Team:TU Munich/Templates/ToggleBoxStart}} | ||
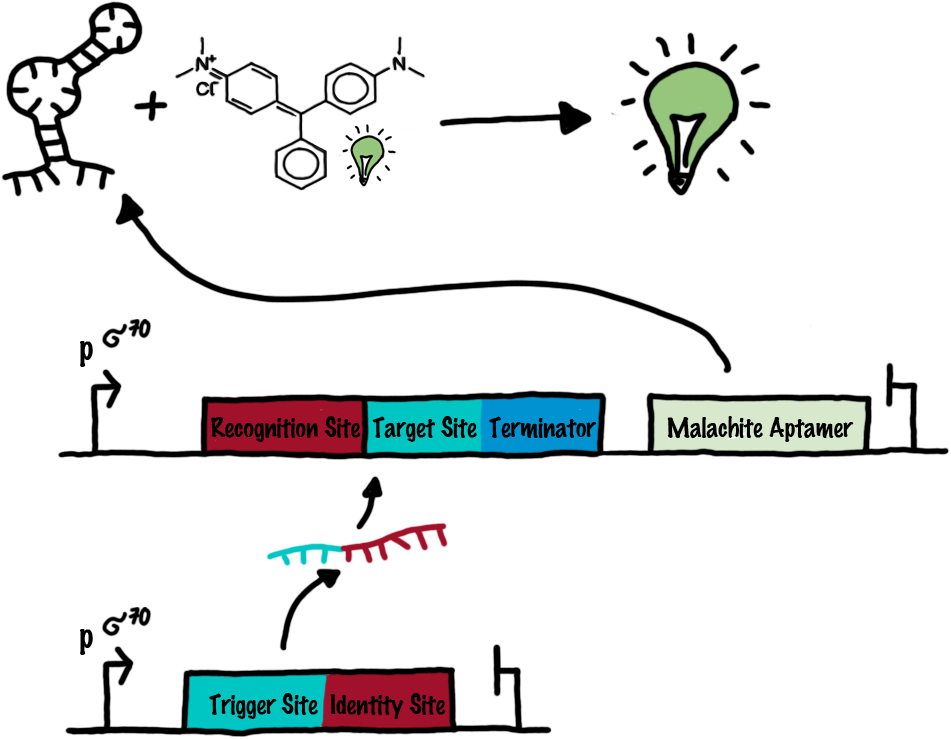
| - | + | A second possibility to measure parameters of our switches we came up with, was the idea to investigate our system on the transcriptional level only. Therefore, we decided to use malachite green as reporter. Malachite green in a fluorescent dye, whose emission increasing dramaticly (about 3000 times) upon binding of a specific RNA-aptamer.<br><br># | |
| + | [[Image:TUM2010_Malachitgruen-2.png|500px|center|our idea]] | ||
| + | ---concept to be desribed, as well as literature--- | ||
| + | <ref>refs</ref> | ||
| + | <br><br> | ||
| + | To study the switches on the transcriptional level gives the advantage, that we would have less interferences and possible artefacts. Also, we are not sure how cellular mechanisms like degradation of RNases or interacting factors as well as molecular crowding influence our systems.<br> | ||
| + | |||
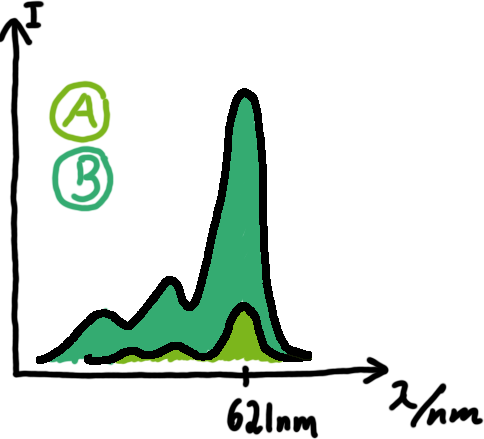
| + | [[Image:TUM2010_Malachit_emission.png|200px|thumb|left|Emission spectra of malachite green; A: without signal-RNA, B: with signal-RNA]]We made constructs comprising of a sigma(70)-binding promoter followed by a short nonsense sequence, the switches and the aptamer sequence.<br>Also we made constructs, where the transcription of the signal-RNA is under the control of a sigma(70) promoter. These two linear DNA-constructs, together with the e.coli RNA-polymerase and the right buffer conditions should represent an easy-to-handle measurement kit on the transcriptional level. | ||
| + | |||
{{:Team:TU Munich/Templates/ToggleBoxEnd}} | {{:Team:TU Munich/Templates/ToggleBoxEnd}} | ||
| + | |||
==In vitro Translation== | ==In vitro Translation== | ||
{{:Team:TU Munich/Templates/ToggleBoxStart}} | {{:Team:TU Munich/Templates/ToggleBoxStart}} | ||
Revision as of 19:30, 10 October 2010
|
||||||||
|
|
Experiment DesignIn vivo Measurements
Our initial idea to prove our concept of antitermination was to use flourescent proteins as reporters. This approach gives the opportunity to measure the termination and antitermination efficiency of our designed BioBricks in vivo as well as in vitro, the latter using a translation kit based on E. coli lysate.
Wiith these measurements, it should also be possible to observe differences in efficiency of termination as well as antitermination between our designed switches. In vitro Measurements
A second possibility to measure parameters of our switches we came up with, was the idea to investigate our system on the transcriptional level only. Therefore, we decided to use malachite green as reporter. Malachite green in a fluorescent dye, whose emission increasing dramaticly (about 3000 times) upon binding of a specific RNA-aptamer. ---concept to be desribed, as well as literature---
<ref>refs</ref>
Also we made constructs, where the transcription of the signal-RNA is under the control of a sigma(70) promoter. These two linear DNA-constructs, together with the e.coli RNA-polymerase and the right buffer conditions should represent an easy-to-handle measurement kit on the transcriptional level. Close In vitro Translation
So haben wir in vivo translation gemessen Close
ProtocolsMolecular BiologyPCR
So geht ne PCR CloseDigestion
So geht ne PCR CloseIn vivo measurement
So haben wir in vivo gemessen CloseIn vitro measurement
So haben wir in vitro gemessen Close
Lab BookExplanation:
|
|||||||
 "
"