Team:TU Delft/Project/alkane-degradation/results
From 2010.igem.org

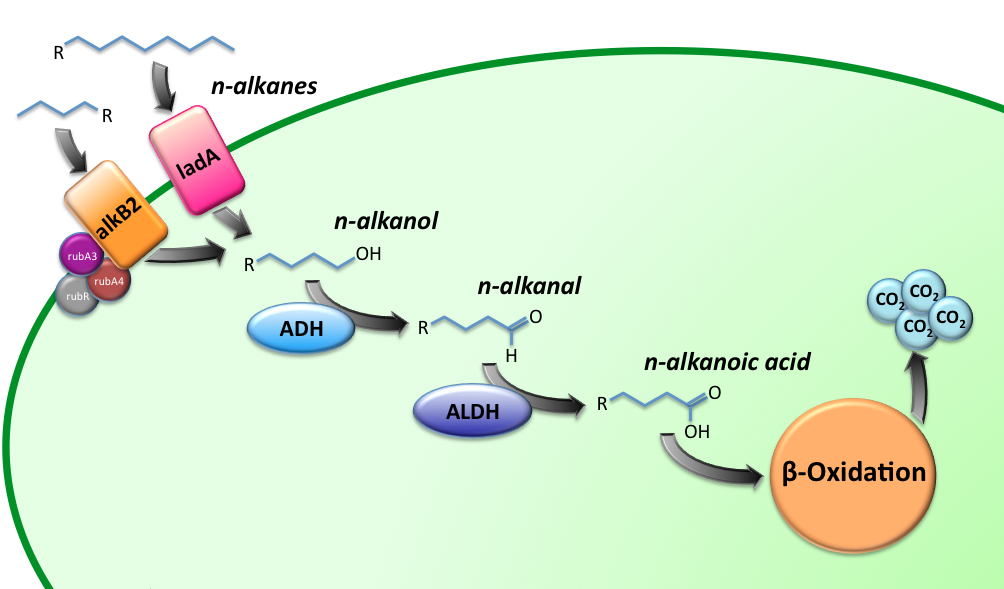
Alkane Degradation Results & Conclusions
Characterization of the alkane hydroxylase system
The following table contains the average ratio of the hexadecane/undecane surface areas. Strains numbers 1 and 2 are two positive colonies taken from the same plate. From the ratios we may conclude that the samples obtained from the E.coli strain, carrying the AH system, contain relatively less octane than the control strain. By comparing peak ratios we were able to estimate the specific enzymatic activity of the system, which was found to be 0.045 U/mg.
For more information about our findings, read the detailed alkane hydroxylase results page.
| Strain | Ratio octane/undecane | Standard deviation [%] | Octane converted [umol] | Protein [mg] | Enzymatic activity [U/mg protein] |
| Blank | 0.832 | 6.16 | 0.00 | 0.00 | 0.00 |
| E.coli K12 negative control | 0.821 | 13.3 | 1.34 | 1.51 | 1.23E-3 |
| E.coli K12 strain #1 (AH-system) | 0.652 | 14.4 | 21.6 | 1.70 | 1.77E-2 |
| E.coli K12 strain #2 (AH-system) | 0.439 | 3.96 | 47.0 | 1.46 | 4.49E-2 |
Characterization of the long-chain alkane monooxygenase; LadA
The analysis of the GC graphs allowed us to estimate the enzymatic activity. The following table displays the average ratios found and the enzyme activity estimates. Strains numbers 1 and 2 are two positive colonies taken from the same plate.
We observed a significant increase in enzyme activity in the strains carrying the ladA protein generator compared to the negative control strain. The highest enzyme activity value was found to be 3.33E-03 U/mg protein. In order to know more about the characterization of this system, read the detailed LadA results page.
| <b>Strain | Average ratio hexadecane/undecane | Standard deviation [%] | Hexadecane converted [umol] | Protein [mg] | Enzymatic activity [U/mg protein] |
| Blank | 0.900 | 3.19 | 0.00 | 0.00 | 0.00 |
| E.coli K12 negative control ([http://partsregistry.org/Part:BBa_J13002 J13002]) | 0.807 | 3.48 | 1.76 | 4.46 | 5.49E-04 |
| E.coli K12 strain #1 ([http://partsregistry.org/Part:BBa_K398017 K398017]) | 0.600 | 15.4 | 5.68 | 4.58 | 1.72E-03 |
| E.coli K12 strain #2 ([http://partsregistry.org/Part:BBa_K398017 K398017]) | 0.532 | 4.25 | 6.95 | 3.18 | 3.03E-03 |
| E.coli TOP10 strain ([http://partsregistry.org/Part:BBa_K398027 K398027]) | 0.492 | 2.98 | 7.71 | 3.22 | 3.33E-03 |
Characterization of the Alcohol DeHydrogenase (ADH) system
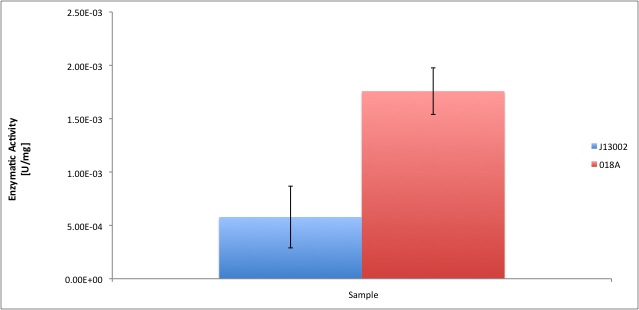
According to our results, the E. coli cell extract has a dodecanol-1 dehydrogenase activity of 9.64e-12 kat/mg (0.58 mU/mg); whereas our recombinant strain expressing the Biobrick [http://partsregistry.org/Part:BBa_K398018 BBa_K398018] has an activity of 2.93e-11 kat/mg (1.76 mU/mg), an improvement of 2-fold compared to the wild type activity; which also means 3% of the activity of the positive control Pseudomonas putida.
If you are interested in knowing more about our findings, read the detailed ADH results page.
Characterization of the ALdehyde DeHydrogenase system
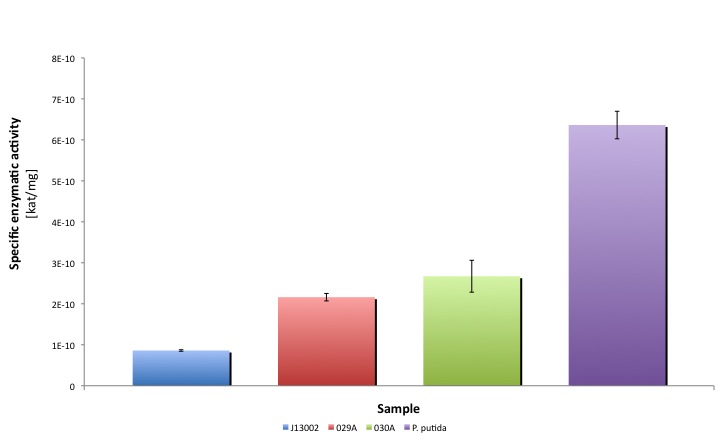
Our results suggest that the recombinant strains E. coli 029A and E. coli 030A functionally express our biobricks. The expression of ALDH under the promoter-rbs combination [http://partsregistry.org/Part:BBa_J13002 BBa_J23100]-[http://partsregistry.org/Part:BBa_J13002 BBa_J61117] increases the dodecanal dehydrogenase activity in E. coli cell extracts 2-fold; whereas the expression of the same protein using the part [http://partsregistry.org/Part:BBa_J13002 BBa_J13002] as promoter-rbs combo increases the same activity 3-fold.
Moreover, the enzymatic activities measured for the constructs [http://partsregistry.org/Part:BBa_K398029 BBa_K398029] and [http://partsregistry.org/Part:BBa_K398030 BBa_K398030] were equivalent to 33.98% and 42.01% of the Pseudomonas putida aldehyde dehydrogenase activity, respectively.
If you want to know more about of our findings, read the detailed ALDH results page.
It is worthy to mention that our part expresses the protein in a lower amount, meaning that cells express a highly active protein. Thus the cellular resources are spent in a more efficient way than in the strain that overproduces ALDH.
Useful Literature and References
- Kato T. et al. "Gene cloning and characterization of an aldehyde dehydrogenase from long-chain alkane-degrading Geobacillus thermoleovorans B23" Extremophiles (2010) 14:33-39.
- http://mbel.kaist.ac.kr/lab/research/protein_en1.html
- Hoffmann F. and Rinas U. "Stress Induced by Recombinant Protein Production in Escherichia coli" Advances in Biochemical Engineering/Biotechnology, 2004, Vol. 89/2004, pp. 73-92.

 "
"