Team:Stockholm/30 August 2010
From 2010.igem.org
(→Andreas) |
|||
| (2 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
{{Stockholm/Top2}} | {{Stockholm/Top2}} | ||
| + | |||
==Andreas== | ==Andreas== | ||
===Cloning of SOD into pMA.His=== | ===Cloning of SOD into pMA.His=== | ||
| Line 25: | Line 26: | ||
====ON cultures==== | ====ON cultures==== | ||
SH1 and SH2 selected for plasmid prep and sequencing. Set 5 ml LB + 100 Amp ON cultures. 37 °C, 225 rpm. | SH1 and SH2 selected for plasmid prep and sequencing. Set 5 ml LB + 100 Amp ON cultures. 37 °C, 225 rpm. | ||
| + | |||
| + | ===N-CPP extraction=== | ||
| + | ====Gel extraction==== | ||
| + | ''From 28/8 samples'' | ||
| + | Purification using the E.Z.N.A. Gel Extraction kit. Elution in 30 μl dH<sub>2</sub>O; double elution. | ||
| + | |||
| + | {|border="1" cellpadding="1" cellspacing="0" | ||
| + | |+ align="bottom"|† ''Phenolate contaminated sample? High absorbtion at 230 nm'' [http://en.wikipedia.org/wiki/Nucleic_acids_analysis#Other_common_contaminants] | ||
| + | !colspan="3"|DNA concentrations | ||
| + | |- | ||
| + | !Sample | ||
| + | !Conc. [ng/μl] | ||
| + | !A<sub>260</sub>/A<sub>280</sub> | ||
| + | |- | ||
| + | |Tra10 | ||
| + | |align="center"|13.56 | ||
| + | |align="center"|1.86 | ||
| + | |- | ||
| + | |TAT | ||
| + | |align="center"|1.736 | ||
| + | |align="center"|1.20 | ||
| + | |- | ||
| + | |LMWP † | ||
| + | |align="center"|2.523 | ||
| + | |align="center"|2.69 | ||
| + | |} | ||
| + | |||
| + | ====Gel verification==== | ||
| + | [[image:Gelver_extr_CPP_30aug.png|200px|thumb|right|'''Gel verification of extracted N-CPPs.'''<br />3 μl λ; 2 μl sample.<br />λ=GeneRuler 50 bp DNA ladder]] | ||
| + | Ran a gel to verify that the presence and size of our extracted DNA fragments. | ||
| + | |||
| + | 1 % agarose, 100 V | ||
| + | |||
| + | '''Results'''<br /> | ||
| + | Weak band for Tra10, no bands for TAT and LMWP. Proceeded to cloning anyway. | ||
| + | |||
| + | ===Cloning of N-CPPs into pSB1C3=== | ||
| + | A last-minute decision was made to also make a bulk cloning of all three N-CPPs by digesting directly from the N-CPP cluster vector. | ||
| + | ====Digestion==== | ||
| + | [N-CPP plasmid]=672 ng/μl (28/8) | ||
| + | {|border="1" cellpadding="1" cellspacing="1" | ||
| + | |align="center"|[ng/μl] | ||
| + | !Tra10 | ||
| + | !TAT | ||
| + | !LMWP | ||
| + | !N-CPP | ||
| + | |- | ||
| + | |10X FD buffer | ||
| + | |align="center"|3 | ||
| + | |align="center"|3 | ||
| + | |align="center"|3 | ||
| + | |align="center"|3 | ||
| + | |- | ||
| + | |dH<sub>2</sub>O | ||
| + | |align="center"|3 | ||
| + | |align="center"|3 | ||
| + | |align="center"|8 | ||
| + | |align="center"|23 | ||
| + | |- | ||
| + | |FD XbaI | ||
| + | |align="center"|0.5 | ||
| + | |align="center"|0.5 | ||
| + | |align="center"|0.5 | ||
| + | |align="center"|0.5 | ||
| + | |- | ||
| + | |FD AgeI | ||
| + | |align="center"|0.5 | ||
| + | |align="center"|0.5 | ||
| + | |align="center"|0.5 | ||
| + | |align="center"|0.5 | ||
| + | |- | ||
| + | |DNA | ||
| + | |align="center"|23 | ||
| + | |align="center"|23 | ||
| + | |align="center"|18 | ||
| + | |align="center"|3 | ||
| + | |- | ||
| + | | | ||
| + | !30 | ||
| + | !30 | ||
| + | !30 | ||
| + | !30 | ||
| + | |} | ||
| + | |||
| + | Incubation: 37 °C, 30 min<br /> | ||
| + | Inactivation: 80 °C, 10 min | ||
| + | |||
| + | =====Dephosphorylation===== | ||
| + | Treated the N-CPP sample with FastAP alkaline phosphatase to prevent multiple insertions (3, 5, etc...) into target vector. | ||
| + | *3 μl FastAP | ||
| + | **Incubation: 37 °C, 10 min | ||
| + | *Inactivation at 75 °C, 5 min | ||
| + | |||
| + | ====Ligation==== | ||
| + | [Dig. pSB1X3 X+A EXTR]=13.72 ng/μl (digested and extracted vector from 9/8) | ||
| + | [Dig. N-CPP X+A]=60 ng/μl | ||
| + | |||
| + | {|border="1" cellpadding="1" cellspacing="0" | ||
| + | |align="center"|[ng/μl] | ||
| + | !N-CPP | ||
| + | !Tra10 | ||
| + | !TAT | ||
| + | !LMWP | ||
| + | |- | ||
| + | |Vector DNA | ||
| + | |align="center"|3 | ||
| + | |align="center"|3 | ||
| + | |align="center"|3 | ||
| + | |align="center"|3 | ||
| + | |- | ||
| + | |Insert DNA | ||
| + | |align="center"|9 | ||
| + | |align="center"|12 | ||
| + | |align="center"|12 | ||
| + | |align="center"|12 | ||
| + | |- | ||
| + | |5X Rapid Lig. buf. | ||
| + | |align="center"|4 | ||
| + | |align="center"|4 | ||
| + | |align="center"|4 | ||
| + | |align="center"|4 | ||
| + | |- | ||
| + | |dH<sub>2</sub>O | ||
| + | |align="center"|3 | ||
| + | |align="center"|0 | ||
| + | |align="center"|0 | ||
| + | |align="center"|0 | ||
| + | |- | ||
| + | |T4 DNA Ligase | ||
| + | |align="center"|1 | ||
| + | |align="center"|1 | ||
| + | |align="center"|1 | ||
| + | |align="center"|1 | ||
| + | |- | ||
| + | | | ||
| + | !20 | ||
| + | !20 | ||
| + | !20 | ||
| + | !20 | ||
| + | |} | ||
| + | |||
| + | ====Transformation==== | ||
| + | Standard transformation protocol. | ||
| + | *2 μl ligation mix | ||
| + | *Cm 25 | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == Mimmi == | ||
| + | |||
| + | === MITF-M === | ||
| + | |||
| + | ==== Colony PCR ==== | ||
| + | |||
| + | |||
| + | *Made by Andreas --> ~280bp bands, empty vector? should not be possible... | ||
| + | |||
| + | *Try again, more colonies | ||
| + | |||
| + | |||
| + | |||
| + | {| | ||
| + | ! Mix | ||
| + | | (µl) | ||
| + | | X8 | ||
| + | | rowspan="8" width="150" | | ||
| + | ! Primers | ||
| + | | rowspan="8" width="150" | | ||
| + | ! colspan="2" | Conditions | ||
| + | | rowspan="3" | | ||
| + | |- | ||
| + | | sH<sub>2</sub>O | ||
| + | | 22.5 | ||
| + | | 180 | ||
| + | | pSB1_VF2 | ||
| + | ! time | ||
| + | ! °C | ||
| + | |- | ||
| + | | F primer | ||
| + | | 1 | ||
| + | | 8 | ||
| + | | pSB1_VR | ||
| + | | 2m | ||
| + | | 94 | ||
| + | |- | ||
| + | | R primer | ||
| + | | 1 | ||
| + | | 8 | ||
| + | | rowspan="5" | | ||
| + | | 30s | ||
| + | | 94 | ||
| + | | ) | ||
| + | |- | ||
| + | | DNA | ||
| + | | 0.5 | ||
| + | | 8x0.5 | ||
| + | | 30s | ||
| + | | 50 | ||
| + | | > 30 cycles | ||
| + | |- | ||
| + | | align="right" | tot | ||
| + | | 25µl | ||
| + | | | ||
| + | | 2m40s | ||
| + | | 72 | ||
| + | | ) | ||
| + | |- | ||
| + | | rowspan="2" colspan="3" | | ||
| + | | 10m | ||
| + | | 72 | ||
| + | | rowspan="2" | | ||
| + | |- | ||
| + | | oo | ||
| + | | 10 | ||
| + | |} | ||
| + | |||
| + | |||
| + | |||
| + | ==== Gel ==== | ||
| + | |||
| + | {| | ||
| + | ! well | ||
| + | ! sample | ||
| + | |- | ||
| + | | 1 | ||
| + | | ladder | ||
| + | |- | ||
| + | | 2 | ||
| + | | pSB1C3.MITF-M 1 | ||
| + | |- | ||
| + | | 3 | ||
| + | | pSB1C3.MITF-M 2 | ||
| + | |- | ||
| + | | 4 | ||
| + | | pSB1C3.MITF-M 3 | ||
| + | |- | ||
| + | | 5 | ||
| + | | pSB1C3.MITF-M 4 | ||
| + | |- | ||
| + | | 6 | ||
| + | | pSB1C3.MITF-M 5 | ||
| + | |- | ||
| + | | 7 | ||
| + | | pSB1C3.MITF-M 6 | ||
| + | |- | ||
| + | | 8 | ||
| + | | pSB1C3.MITF-M 7 | ||
| + | |- | ||
| + | | 9 | ||
| + | | blank | ||
| + | |} | ||
| + | |||
| + | |||
| + | *No product, trying another 7 colonies over night... | ||
| + | |||
| + | {{Stockholm/Footer}} | ||
Latest revision as of 11:03, 26 October 2010
Contents |
Andreas
Cloning of SOD into pMA.His
Transformation results
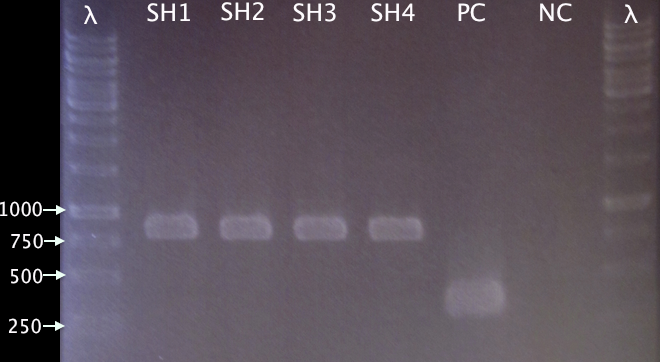
From 28 28/8 Good colony yield. Four colonies (SH1-SH4) picked for colony PCR.
Colony PCR
- SH1-SH4: pMA.SOD⋅His
- PC: Positive control; pMA.His
- NC: Negative control; blank
Procedures according to standard colony PCR protocol. Elongation time 1:00.
Gel verification
1 % agarose, 100 V
Expected bands:
- pMA.SOD⋅His: 831 bp
- pMA.His: 348 bp
Results
Well corresponding bands indicating successful insertion of SOD into the vector.
ON cultures
SH1 and SH2 selected for plasmid prep and sequencing. Set 5 ml LB + 100 Amp ON cultures. 37 °C, 225 rpm.
N-CPP extraction
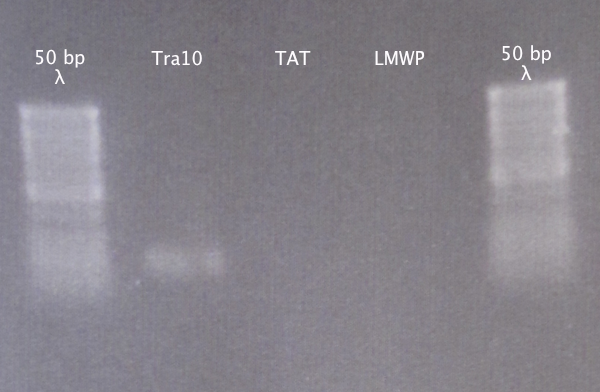
Gel extraction
From 28/8 samples Purification using the E.Z.N.A. Gel Extraction kit. Elution in 30 μl dH2O; double elution.
| DNA concentrations | ||
|---|---|---|
| Sample | Conc. [ng/μl] | A260/A280 |
| Tra10 | 13.56 | 1.86 |
| TAT | 1.736 | 1.20 |
| LMWP † | 2.523 | 2.69 |
Gel verification
Ran a gel to verify that the presence and size of our extracted DNA fragments.
1 % agarose, 100 V
Results
Weak band for Tra10, no bands for TAT and LMWP. Proceeded to cloning anyway.
Cloning of N-CPPs into pSB1C3
A last-minute decision was made to also make a bulk cloning of all three N-CPPs by digesting directly from the N-CPP cluster vector.
Digestion
[N-CPP plasmid]=672 ng/μl (28/8)
| [ng/μl] | Tra10 | TAT | LMWP | N-CPP |
|---|---|---|---|---|
| 10X FD buffer | 3 | 3 | 3 | 3 |
| dH2O | 3 | 3 | 8 | 23 |
| FD XbaI | 0.5 | 0.5 | 0.5 | 0.5 |
| FD AgeI | 0.5 | 0.5 | 0.5 | 0.5 |
| DNA | 23 | 23 | 18 | 3 |
| 30 | 30 | 30 | 30 |
Incubation: 37 °C, 30 min
Inactivation: 80 °C, 10 min
Dephosphorylation
Treated the N-CPP sample with FastAP alkaline phosphatase to prevent multiple insertions (3, 5, etc...) into target vector.
- 3 μl FastAP
- Incubation: 37 °C, 10 min
- Inactivation at 75 °C, 5 min
Ligation
[Dig. pSB1X3 X+A EXTR]=13.72 ng/μl (digested and extracted vector from 9/8) [Dig. N-CPP X+A]=60 ng/μl
| [ng/μl] | N-CPP | Tra10 | TAT | LMWP |
|---|---|---|---|---|
| Vector DNA | 3 | 3 | 3 | 3 |
| Insert DNA | 9 | 12 | 12 | 12 |
| 5X Rapid Lig. buf. | 4 | 4 | 4 | 4 |
| dH2O | 3 | 0 | 0 | 0 |
| T4 DNA Ligase | 1 | 1 | 1 | 1 |
| 20 | 20 | 20 | 20 |
Transformation
Standard transformation protocol.
- 2 μl ligation mix
- Cm 25
Mimmi
MITF-M
Colony PCR
- Made by Andreas --> ~280bp bands, empty vector? should not be possible...
- Try again, more colonies
| Mix | (µl) | X8 | Primers | Conditions | ||||
|---|---|---|---|---|---|---|---|---|
| sH2O | 22.5 | 180 | pSB1_VF2 | time | °C | |||
| F primer | 1 | 8 | pSB1_VR | 2m | 94 | |||
| R primer | 1 | 8 | 30s | 94 | ) | |||
| DNA | 0.5 | 8x0.5 | 30s | 50 | > 30 cycles | |||
| tot | 25µl | 2m40s | 72 | ) | ||||
| 10m | 72 | |||||||
| oo | 10 | |||||||
Gel
| well | sample |
|---|---|
| 1 | ladder |
| 2 | pSB1C3.MITF-M 1 |
| 3 | pSB1C3.MITF-M 2 |
| 4 | pSB1C3.MITF-M 3 |
| 5 | pSB1C3.MITF-M 4 |
| 6 | pSB1C3.MITF-M 5 |
| 7 | pSB1C3.MITF-M 6 |
| 8 | pSB1C3.MITF-M 7 |
| 9 | blank |
- No product, trying another 7 colonies over night...
 |

|
 |

|
 |

|

|

|
 "
"