Team:SDU-Denmark/project-p
From 2010.igem.org
(Difference between revisions)
(→K343005) |
(→K343007) |
||
| Line 54: | Line 54: | ||
There is a wide range of motility assays for chemotaxis in bacteria, which meant that we had a broad spectrum of experiments to choose from, which just had to be tweaked for making them suited for the analysis of phototaxis. The two experiments we chose for analysing the effect of this part (PS), were growth of the bacterial cultures in semi-solid agar and computer analysis of swimming motility through video microscopy:<br> | There is a wide range of motility assays for chemotaxis in bacteria, which meant that we had a broad spectrum of experiments to choose from, which just had to be tweaked for making them suited for the analysis of phototaxis. The two experiments we chose for analysing the effect of this part (PS), were growth of the bacterial cultures in semi-solid agar and computer analysis of swimming motility through video microscopy:<br> | ||
<br> | <br> | ||
| - | + | === Growth of bacterial culture on semi-solid agar plates=== | |
| + | '''Experiment 1'''<br> | ||
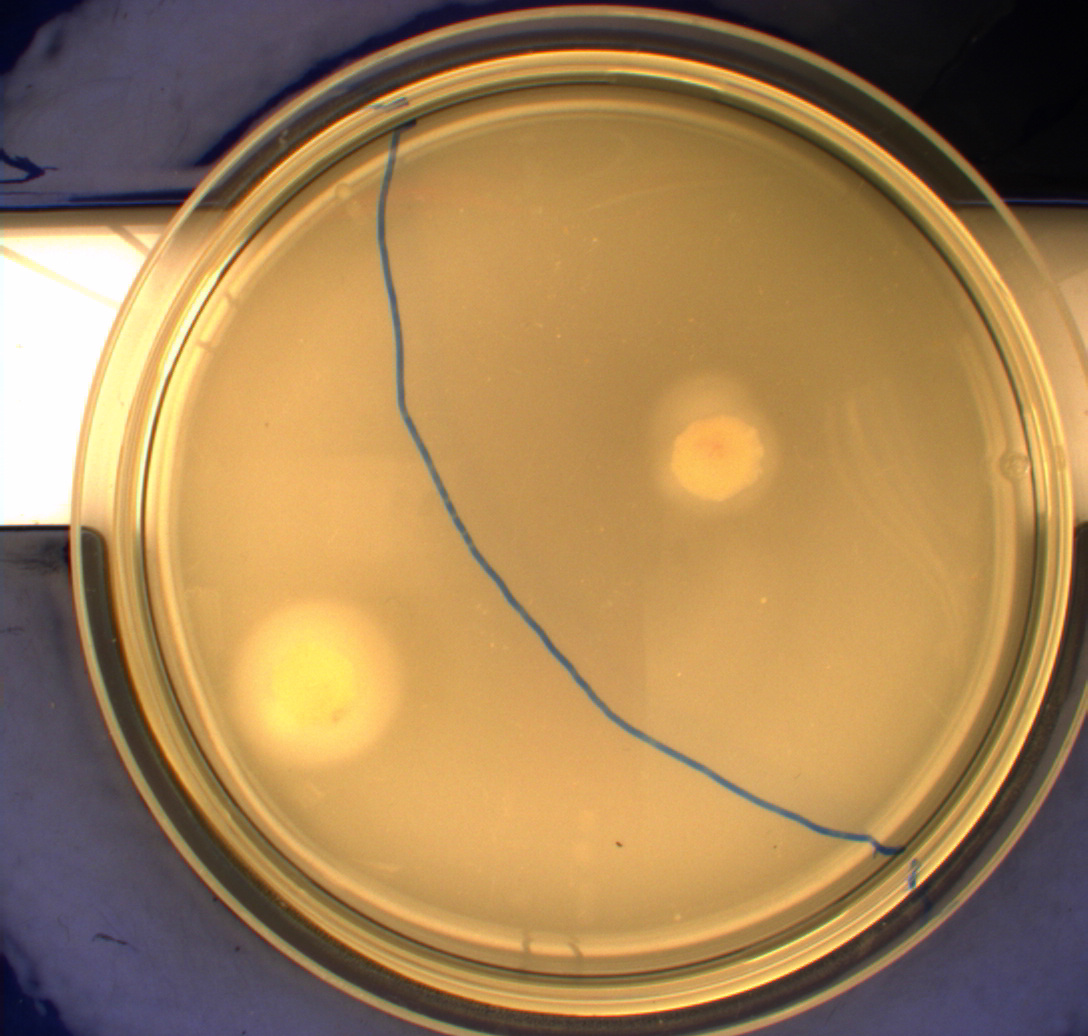
The bacteria and plates were prepared after our own protocol, which you can find here: [https://2010.igem.org/Team:SDU-Denmark/protocols#Photosensor_characterisation PS1.1] <br> [[Image:Ingeniør1.JPG|200px|thumb|left|Wildtype bacteria (Left half exposed to light, right half exposed to darkness)]][[Image:Ingeniør4.JPG|200px|thumb|left|Phototactic bacteria (Left half exposed to light, right half exposed to darkness)]] | The bacteria and plates were prepared after our own protocol, which you can find here: [https://2010.igem.org/Team:SDU-Denmark/protocols#Photosensor_characterisation PS1.1] <br> [[Image:Ingeniør1.JPG|200px|thumb|left|Wildtype bacteria (Left half exposed to light, right half exposed to darkness)]][[Image:Ingeniør4.JPG|200px|thumb|left|Phototactic bacteria (Left half exposed to light, right half exposed to darkness)]] | ||
Since exposure to blue light should decrease the phototaxic bacterias tumbling frequency, the expected result was that the colony which was placed between the light and dark half of the plate would spread out in the darkness and would not move further when it reached the light. This is counter-intuitive, since decreased tumbling should lead to a longer distance traveled. What happens at the microscopic scale in semisolid agar is that the agar creates a matrix like structure where there are channels through the agar, which the bacteria can swim through. The decrease in tumbling frequency of the bacteria will make it harder for them to find the channels in the agar to swim through, which leads to them being trapped where they were placed. The result is that a colony which shows an increased run time, will look as if it it was non-motile on these plates. Our results showed exactly this; the bacterial culture had spread out to on the dark half of the plate and did not get nearly as far on the half exposed to light. This experiment was done with a normal wildtype MG1655 and a non-motile strain of ''E.coli'', DH5alpha, as controls. As expected these cells did not show anything like the behavior described above, which indicates that the effect stems from the modification to our photosensor bacteria. These results were useable, but not fully conclusive, since there were some non-optimal conditions present in this experiment. We used ambient light instead of pure blue light, and the exposure to light for the multiple samples was not exactly even. Therefore we had to improve our experiment setup and see if we could reproduce these results with a more reliable setup.<br> | Since exposure to blue light should decrease the phototaxic bacterias tumbling frequency, the expected result was that the colony which was placed between the light and dark half of the plate would spread out in the darkness and would not move further when it reached the light. This is counter-intuitive, since decreased tumbling should lead to a longer distance traveled. What happens at the microscopic scale in semisolid agar is that the agar creates a matrix like structure where there are channels through the agar, which the bacteria can swim through. The decrease in tumbling frequency of the bacteria will make it harder for them to find the channels in the agar to swim through, which leads to them being trapped where they were placed. The result is that a colony which shows an increased run time, will look as if it it was non-motile on these plates. Our results showed exactly this; the bacterial culture had spread out to on the dark half of the plate and did not get nearly as far on the half exposed to light. This experiment was done with a normal wildtype MG1655 and a non-motile strain of ''E.coli'', DH5alpha, as controls. As expected these cells did not show anything like the behavior described above, which indicates that the effect stems from the modification to our photosensor bacteria. These results were useable, but not fully conclusive, since there were some non-optimal conditions present in this experiment. We used ambient light instead of pure blue light, and the exposure to light for the multiple samples was not exactly even. Therefore we had to improve our experiment setup and see if we could reproduce these results with a more reliable setup.<br> | ||
<br> | <br> | ||
| - | ''' | + | '''Experiment 2''' |
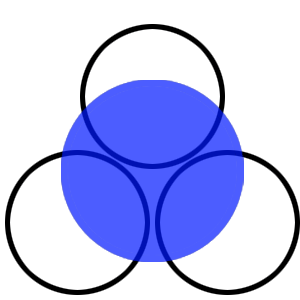
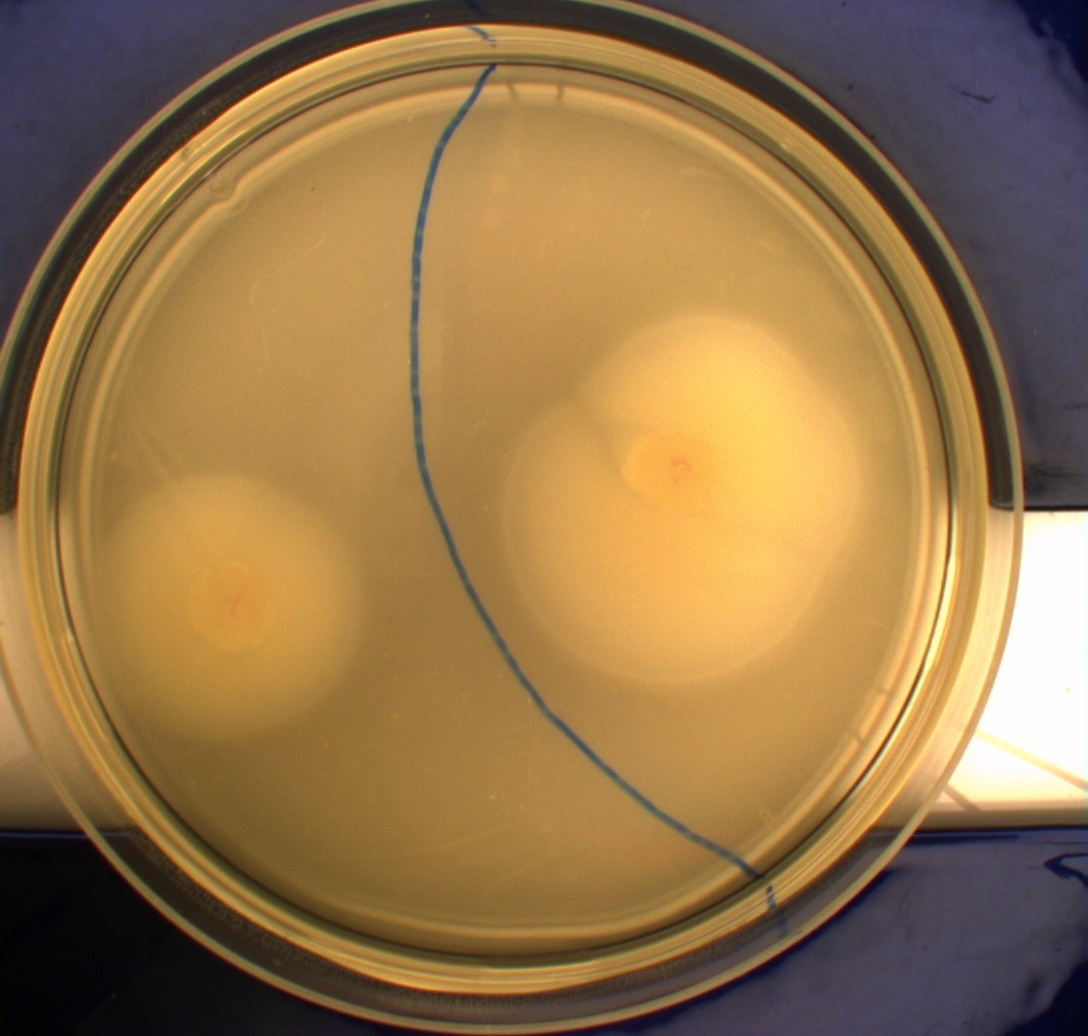
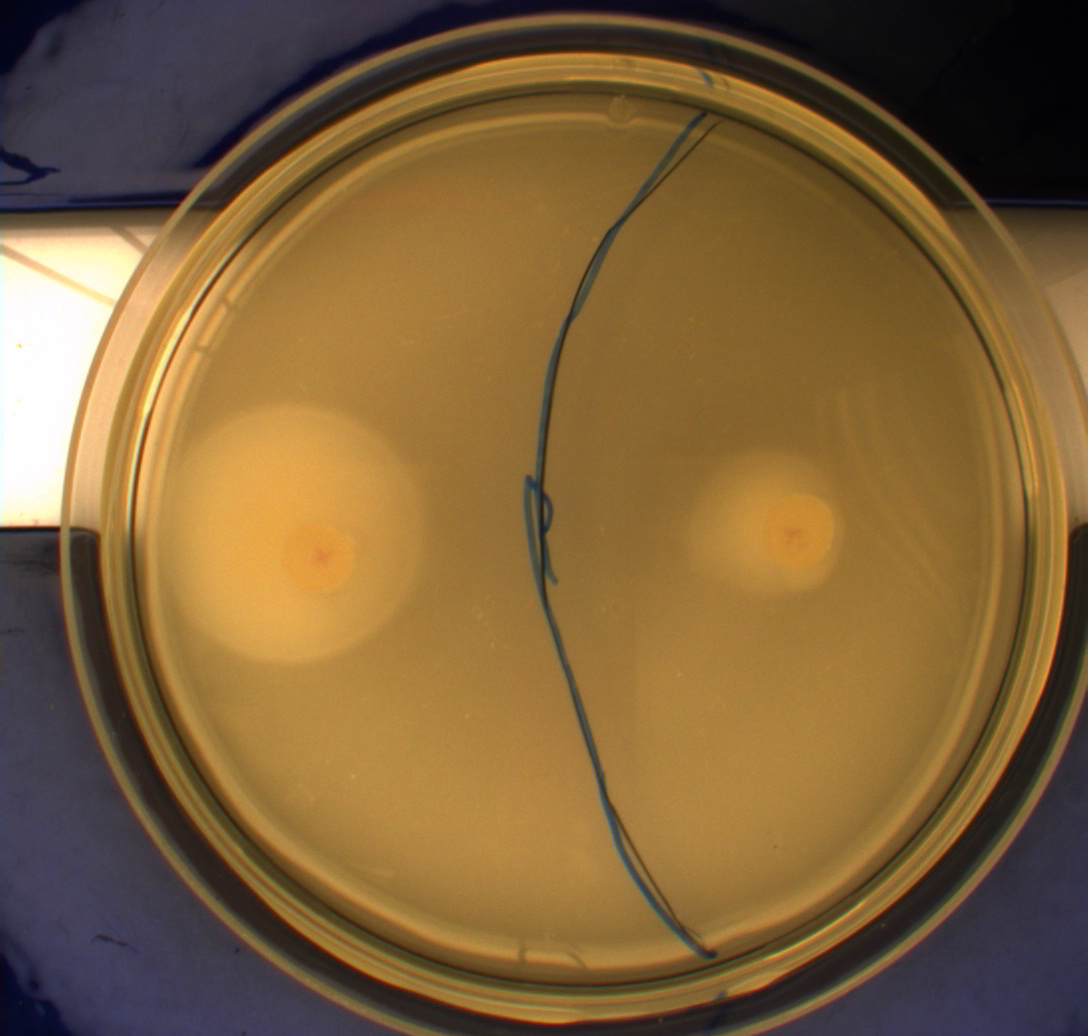
The experiment described above was repeated in a more controlled environment. This means that there were no changes as to how the plates or bacterial cultures were prepared, so we refer again to [https://2010.igem.org/Team:SDU-Denmark/protocols#Photosensor_characterisation PS1.1] in regards to how this was done. The difference lies in the setup of the light-controlled environment. What we did this time was that the plates was illuminated from above by a single light source in an otherwise completely dark environment, we prepared a cut-out so that the light would only hit one half of our plates and the other half would remain in the dark. [[Image:Lightbox5k.gif|thumb|Schematic over plate positioning and areas exposed to light.]] Since the problem with the last experiment was that the light source was just normal white light (which contains a lot of different wavelengths), this time around we used an optical filter so that only light with a wavelength of around 470nm could pass through, which resulted in a blue light shining down on the plates. The light-source itself was a run-of-the-mill flashlight, with a blue light filter installed in front of the lens. To eliminate the effect of temperature gradients inside the incubator, the three samples were placed in a triangle formation in the center of the incubator. <br> | The experiment described above was repeated in a more controlled environment. This means that there were no changes as to how the plates or bacterial cultures were prepared, so we refer again to [https://2010.igem.org/Team:SDU-Denmark/protocols#Photosensor_characterisation PS1.1] in regards to how this was done. The difference lies in the setup of the light-controlled environment. What we did this time was that the plates was illuminated from above by a single light source in an otherwise completely dark environment, we prepared a cut-out so that the light would only hit one half of our plates and the other half would remain in the dark. [[Image:Lightbox5k.gif|thumb|Schematic over plate positioning and areas exposed to light.]] Since the problem with the last experiment was that the light source was just normal white light (which contains a lot of different wavelengths), this time around we used an optical filter so that only light with a wavelength of around 470nm could pass through, which resulted in a blue light shining down on the plates. The light-source itself was a run-of-the-mill flashlight, with a blue light filter installed in front of the lens. To eliminate the effect of temperature gradients inside the incubator, the three samples were placed in a triangle formation in the center of the incubator. <br> | ||
Another deviation from the protocol PS1.1 is that the culture was inoculated at the center of the plate, instead one colony each was inoculated in the center of the light exposed and dark half respectively. This would give us more information on how the bacteria would behave when directly exposed to either the dark or light surroundings, instead of the gradient that was present in the first experiment.<br> | Another deviation from the protocol PS1.1 is that the culture was inoculated at the center of the plate, instead one colony each was inoculated in the center of the light exposed and dark half respectively. This would give us more information on how the bacteria would behave when directly exposed to either the dark or light surroundings, instead of the gradient that was present in the first experiment.<br> | ||
| Line 67: | Line 68: | ||
<br> | <br> | ||
| - | + | === Videomicroscopy and computer analysis of bacterial motility ==== | |
| + | '''Experiment 1'''<br> | ||
To get an exact idea of what is happening to the bacteria when expressing the photosensor we decided on doing video microscopy of motile bacteria. The bacterial cultures were prepared acording to protocol [https://2010.igem.org/Team:SDU-Denmark/protocols#PS1.1 PS1.1]. Wildtype MG1655 and DH5alpha was used as controls. | To get an exact idea of what is happening to the bacteria when expressing the photosensor we decided on doing video microscopy of motile bacteria. The bacterial cultures were prepared acording to protocol [https://2010.igem.org/Team:SDU-Denmark/protocols#PS1.1 PS1.1]. Wildtype MG1655 and DH5alpha was used as controls. | ||
The actual microscopy was done on a (insert name) microscope with an optical magnification of 1000x. <br> | The actual microscopy was done on a (insert name) microscope with an optical magnification of 1000x. <br> | ||
| Line 87: | Line 89: | ||
This might indicate a decreased tumbling frequency, but the low motility of the bacteria, the inadequacy of the cell track software and the small amount of data analysed make results of this experiment unreliable and a new protocol had to be devised.<br><br> | This might indicate a decreased tumbling frequency, but the low motility of the bacteria, the inadequacy of the cell track software and the small amount of data analysed make results of this experiment unreliable and a new protocol had to be devised.<br><br> | ||
| - | + | ===Computerized analysis of the bacterial motility with the THOR prototype by [http://unisensor.dk/ Unisensor A/S] and the Unify software=== | |
For this experiment we changed our protocol for cultivating swimming bacteria in order to optimize their mobility [https://2010.igem.org/Team:SDU-Denmark/protocols#PS1.2 PS1.2]<br> | For this experiment we changed our protocol for cultivating swimming bacteria in order to optimize their mobility [https://2010.igem.org/Team:SDU-Denmark/protocols#PS1.2 PS1.2]<br> | ||
The machine and software used for the analysis are prototypes and still under heavy development. Because of trade secrets it is impossible for us to explain how the machine works or give a detailed explanation of it's mechanism until the THOR has reached production status. Simplistically said it is a very advanced video microscope, with which it is possible to analyse liquids and the particles inside in both 2D and 3D, while also tracking them over time. | The machine and software used for the analysis are prototypes and still under heavy development. Because of trade secrets it is impossible for us to explain how the machine works or give a detailed explanation of it's mechanism until the THOR has reached production status. Simplistically said it is a very advanced video microscope, with which it is possible to analyse liquids and the particles inside in both 2D and 3D, while also tracking them over time. | ||
Revision as of 19:42, 25 October 2010
 "
"