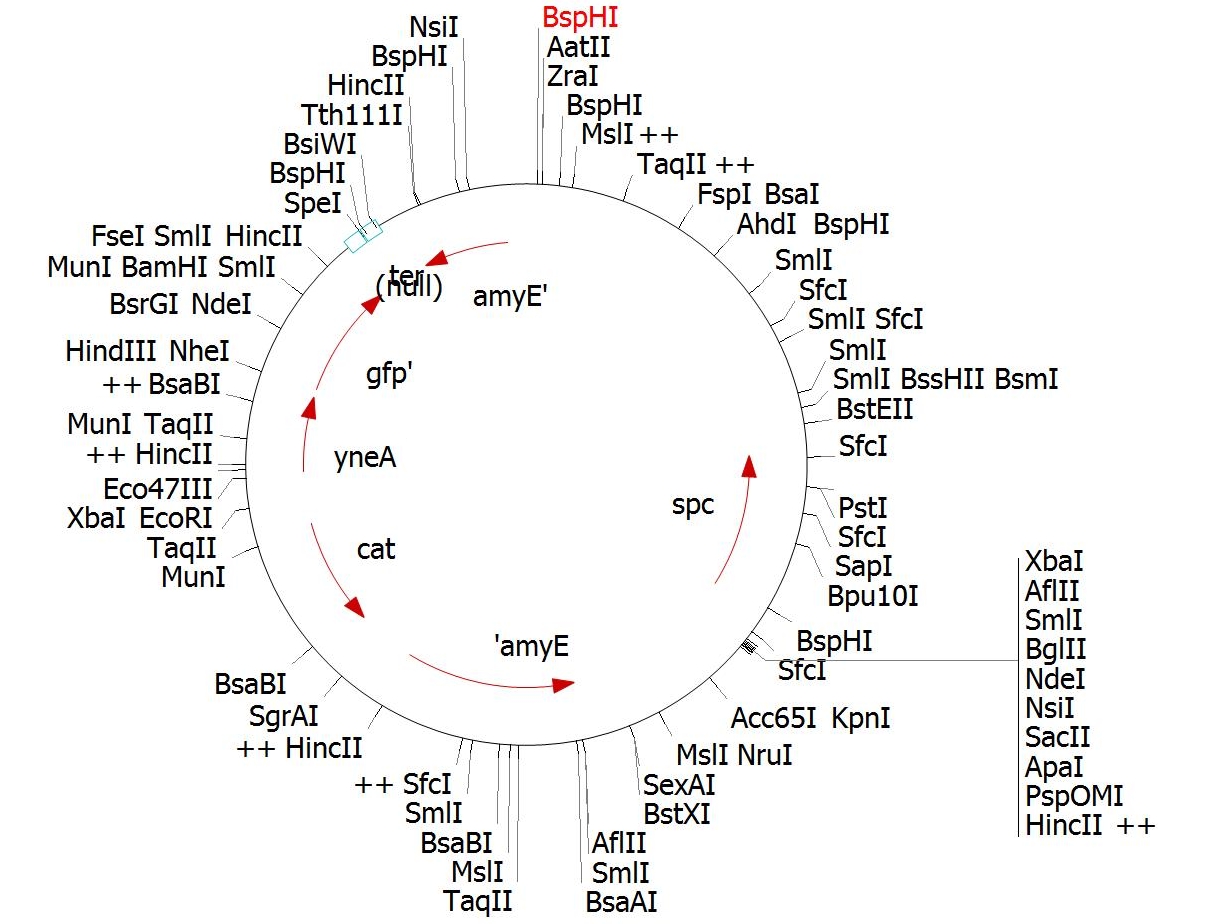
|
|
| (14 intermediate revisions not shown) |
| Line 1: |
Line 1: |
| | {{Team:Newcastle/mainbanner}} | | {{Team:Newcastle/mainbanner}} |
| | | | |
| - | =Gel exlectrophoresis for single digestion of pSB1C3=
| |
| | | | |
| - | ==Aim==
| |
| | | | |
| - | The aim of this experiment is to check if the digestion from [[Team:Newcastle/24_August_2010|yesterday]] worked.
| + | =Transformation of ''Bacillius subtilis'' 168 with pGFP-rrnB containing ''yneA''= |
| | | | |
| - | ==Materials and Protocol== | + | ==Aim== |
| | | | |
| - | Please refer to [[Team:Newcastle/Gel_electrophoresis|gel electrophoresis]] for protocol.
| + | We performed gel electrophoresis on our 12 digested minipreps and found 8 were the correct product. We transformed ''B. subtilis'' 168 with one of the samples (1). |
| | | | |
| - | ==Results==
| + | [[Image:filamentous_in_pgfprrnb.jpg|800px]] |
| - | | + | |
| - | The result from gel electrophoresis:
| + | |
| - | [[Image:Newcastle 25-08-2010 – Gel 1 (Ethidium Bromide).png|500px|centre]] | + | |
| - | '''Figure 1''' shows the double digest of 12 tubes of pGFPrrnB and ''yneA''.
| + | |
| - | | + | |
| - | *'''Lane 1''': Vector only
| + | |
| - | *'''Lane 2''': Tube 1
| + | |
| - | *'''Lane 3''': Tube 2
| + | |
| - | *'''Lane 4''': Tube 3
| + | |
| - | *'''Lane 5''': Tube 4
| + | |
| - | *'''Lane 6''': Tube 5
| + | |
| - | *'''Lane 7''': Tube 6
| + | |
| - | *'''Lane 8''': Tube 7
| + | |
| - | *'''Lane 9''': Tube 8
| + | |
| - | *'''Lane 10''': Tube 9
| + | |
| - | *'''Lane 11''': Tube 10
| + | |
| - | *'''Lane 12''': Tube 11
| + | |
| - | *'''Lane 13''': Tube 12
| + | |
| - | | + | |
| - | ===Discussion===
| + | |
| - | | + | |
| - | The bands we got from the gel shows that digestion in tubes 1, 2, 4, 10, 11 and 12 worked.
| + | |
| - | | + | |
| - | ===Conclusion===
| + | |
| - | | + | |
| - | We use the digested products from the six tubes that worked for ligation.
| + | |
| - | | + | |
| - | | + | |
| - | =Overnight culture for transformation of ''B. subtilis'' with ''yneA''=
| + | |
| - | | + | |
| - | ==Aim==
| + | |
| - | | + | |
| - | To transform ''yneA'' into competent ''B. subtilis''.
| + | |
| | | | |
| | ==Materials and Protocol== | | ==Materials and Protocol== |
| Line 54: |
Line 19: |
| | Please refer to results in [[Team:Newcastle/26_August_2010|tomorrow]]'s lab book. | | Please refer to results in [[Team:Newcastle/26_August_2010|tomorrow]]'s lab book. |
| | | | |
| - | ==Transformation==
| |
| - |
| |
| - | ===Aim===
| |
| - |
| |
| - | To transform pGFPrrnB and ''yneA'' into E. coli.
| |
| - |
| |
| - | ===Materials and Protocol===
| |
| - |
| |
| - | Please refer to [[Team:Newcastle/Transformation_of_E._coli|transformation of E. coli]].
| |
| - |
| |
| - | ===Results and Conclusion===
| |
| - |
| |
| - | Please refer to [[Team:Newcastle/26_August_2010|tomorrow]]'s lab book.
| |
| - |
| |
| - |
| |
| - | ==PCR==
| |
| - |
| |
| - | ===Aim===
| |
| - |
| |
| - | To amplify the DNA pSB1C3 using RocF primer.
| |
| - |
| |
| - | ===Materials and Protocol===
| |
| - |
| |
| - | Please refer to [[Team:Newcastle/PCR|PCR]].
| |
| - |
| |
| - | ===Conclusion===
| |
| - |
| |
| - | Continue with PCR purification.
| |
| - |
| |
| - |
| |
| - | ==PCR Purification==
| |
| - |
| |
| - | ===Aim===
| |
| - |
| |
| - | To remove unwanted primers, taq polymerase, buffer and salts to obtain pure DNA.
| |
| - |
| |
| - | ===Materials and Protocol===
| |
| - |
| |
| - | Please refer to [[Team:Newcastle/PCR_purification|PCR purification]].
| |
| - |
| |
| - |
| |
| - | ==Digestion==
| |
| - |
| |
| - | ===Aim===
| |
| - |
| |
| - | To digest the PCR products of pSB1C3 and ''yneA'' from PCR purification.
| |
| - |
| |
| - | ===Materials and Protocol===
| |
| - |
| |
| - | Please refer to [[Team:Newcastle/Restriction_digests|restriction digest]].
| |
| - |
| |
| - |
| |
| - | ==Gel extraction==
| |
| - |
| |
| - | ===Aim===
| |
| - |
| |
| - | To purify the DNA of ''yneA'' and pSB1C3 by extracting the bands from the gel after running gel electrophoresis. Concentration of DNA is then checked with NanoDrop.
| |
| - |
| |
| - | ===Materials and Protocol===
| |
| - |
| |
| - | Please refer to:
| |
| - |
| |
| - | * [[Team:Newcastle/Gel_electrophoresis|gel electrophoresis]],
| |
| - | * [[Team:Newcastle/Gel_extraction|gel extraction]] and
| |
| - | * [[TeamNewcastleNanoDrop_Spectrophotometer|nanodrop spectrophotometer]].
| |
| - |
| |
| - | ===Results===
| |
| - |
| |
| - | The bands we got from gel electrophoresis is very faint.
| |
| - |
| |
| - | ===Conclusion===
| |
| - |
| |
| - | We realized that we used the wrong ''rocF'' primer, so we repeat the whole procedure from PCR again.
| |
| - |
| |
| - | =Restriction digestion and gel extraction linearized pSB1C3=
| |
| - |
| |
| - | ==Aims==
| |
| - |
| |
| - | The aim of this experiment is to digested the plasmid pSB1C3 with the restriction enzyme HindIII to linearize it and and to perform gel extraction to purify it.
| |
| - |
| |
| - | ==Materials and protocol==
| |
| - |
| |
| - | Please refer to the:
| |
| - | *[[Team:Newcastle/Gel_electrophoresis| gel electrophoresis]],
| |
| - | *[[Team:Newcastle/Gel_extraction| gel extraction]] and
| |
| - | *[[TeamNewcastleNanoDrop_Spectrophotometer| NanoDrop spectrophotometer]] protocols.
| |
| - |
| |
| - | ==Results==
| |
| - |
| |
| - | *'''Lane 1''': 1 Kb ladder
| |
| - | *'''Lane 2''': Linearized plasmid pSB1C3
| |
| - | *'''Lane 3''': 1 Kb ladder
| |
| - |
| |
| - | There is no gel photograph because we want to keep the exposure of DNA to the UV light to an absolute minimum.
| |
| - |
| |
| - | ==Discussion==
| |
| - |
| |
| - | During gel extraction procedure, we found a bright band of approx
| |
| - | During gel extraction procedure, we found a bright band of approximately 3100 bp size in lane 2 under UV light and we cut the gel and extracted the band.
| |
| - |
| |
| - | ==Conclusion==
| |
| - |
| |
| - | We got linearized plasmid pSB1C3 and we performed gel extraction successfully and the nanodrop protocol showed that we got 12.7 ng/µl concentration of plasmid.
| |
| | | | |
| | {{Team:Newcastle/footer}} | | {{Team:Newcastle/footer}} |



 "
"