Team:Kyoto/Notebook1
From 2010.igem.org
| Line 3: | Line 3: | ||
===Construction for Lysisbox=== | ===Construction for Lysisbox=== | ||
====Tuesday, July 20 <span class="by">By: Wataru, Tomo, Yuki, Kazuya, Ken, Makoto</span>==== | ====Tuesday, July 20 <span class="by">By: Wataru, Tomo, Yuki, Kazuya, Ken, Makoto</span>==== | ||
| - | ===== | + | =====Transformation===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Well||Sample||Competent Cells||Total||Plate||Incubation||Colony | !Name||Well||Sample||Competent Cells||Total||Plate||Incubation||Colony | ||
| Line 28: | Line 28: | ||
====Wednesday, July 21 <span class="by">By: Wataru, Ken, Makoto, Takuya Y.</span>==== | ====Wednesday, July 21 <span class="by">By: Wataru, Ken, Makoto, Takuya Y.</span>==== | ||
=====Culture at 37℃ from 07/21 20:50 to 07/22 17:00 and Making Master Plate===== | =====Culture at 37℃ from 07/21 20:50 to 07/22 17:00 and Making Master Plate===== | ||
| - | ===== | + | =====Transformation===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Well||Sample||Competent Cells||Total||Plate||Incubation||Colony | !Name||Well||Sample||Competent Cells||Total||Plate||Incubation||Colony | ||
| Line 36: | Line 36: | ||
|B0015||1-23-L||1||20||21||○ | |B0015||1-23-L||1||20||21||○ | ||
|} | |} | ||
| - | ===== | + | =====PCR for SRRz and S===== |
{| class="experiments" | {| class="experiments" | ||
!No.||Water||MgSO4||dNTPs||10xBuffer||Template DNA||Primer Fwd.||Primer Rev. (SRRz)||Primer Rev. (S)||KOD Plus ver.2||Total | !No.||Water||MgSO4||dNTPs||10xBuffer||Template DNA||Primer Fwd.||Primer Rev. (SRRz)||Primer Rev. (S)||KOD Plus ver.2||Total | ||
| Line 71: | Line 71: | ||
====Thursday, July 22 <span class="by">By: Wataru</span>==== | ====Thursday, July 22 <span class="by">By: Wataru</span>==== | ||
| - | ===== | + | =====Electrophoresis (40min) of the PCR Products===== |
[[Image:KyotoExp100722-1.png|300px|right]] | [[Image:KyotoExp100722-1.png|300px|right]] | ||
{| class="electrophoresis" | {| class="electrophoresis" | ||
| Line 93: | Line 93: | ||
|} | |} | ||
Marker: 100bp, 1kb, 1kb, 100bp. | Marker: 100bp, 1kb, 1kb, 100bp. | ||
| - | ===== | + | =====Miniprep===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Concentration | !Name||Concentration | ||
| Line 114: | Line 114: | ||
====Friday, July 23 <span class="by">By: Wataru, Tomo, Makoto</span>==== | ====Friday, July 23 <span class="by">By: Wataru, Tomo, Makoto</span>==== | ||
| - | ===== | + | =====Miniprep===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Concentration | !Name||Concentration | ||
| Line 123: | Line 123: | ||
|} | |} | ||
We lost B0015 by our mistake. The concentration of pSB4K5 is high, so this condition of shaking incubation is moderate. | We lost B0015 by our mistake. The concentration of pSB4K5 is high, so this condition of shaking incubation is moderate. | ||
| - | ===== | + | =====PCR Purification===== |
{| class="experiments" | {| class="experiments" | ||
!No.||Name||Concentration||New Name | !No.||Name||Concentration||New Name | ||
| Line 136: | Line 136: | ||
|} | |} | ||
The concentration of sample number 1 and 5, the PCR products of S-R-Rz/Rz1, is week, so we desided to retry PCR. | The concentration of sample number 1 and 5, the PCR products of S-R-Rz/Rz1, is week, so we desided to retry PCR. | ||
| - | ===== | + | =====PCR for SRRz===== |
{| class="experiments" | {| class="experiments" | ||
!No.||Water||MgSO4||dNTPs||10xBuffer||Template DNA||Primer Fwd. (SRRz)||Primer Rev. (SRRz)||KOD plus ver.2||Total | !No.||Water||MgSO4||dNTPs||10xBuffer||Template DNA||Primer Fwd. (SRRz)||Primer Rev. (SRRz)||KOD plus ver.2||Total | ||
| Line 164: | Line 164: | ||
|4℃||forever|| | |4℃||forever|| | ||
|} | |} | ||
| - | ===== | + | =====Restriction Digestion and Electrophoresis (35min) to check function of our Restriction Enzyme===== |
{| class="experiments" | {| class="experiments" | ||
!No.||Name||Sample||10xBuffer||BSA||colspan="2"|Enzyme||MilliQ||Total||Incubation | !No.||Name||Sample||10xBuffer||BSA||colspan="2"|Enzyme||MilliQ||Total||Incubation | ||
| Line 181: | Line 181: | ||
Marker: 1kb. | Marker: 1kb. | ||
Comparison to No. 5 (control, circular DNA), the bands of No. 1, 2, 3, and 4 was shifted. The DNA of them was linearized by Restriction enzymes. So, our restriction enzymes work correctly. | Comparison to No. 5 (control, circular DNA), the bands of No. 1, 2, 3, and 4 was shifted. The DNA of them was linearized by Restriction enzymes. So, our restriction enzymes work correctly. | ||
| - | ===== | + | =====Restriction Digestion and Ligation to insert S gene to E0840===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Sample||10xBuffer||colspan="2"|Enzyme 1||colspan="2"|Enzyme 2||MilliQ||Total||Incubation | !Name||Sample||10xBuffer||colspan="2"|Enzyme 1||colspan="2"|Enzyme 2||MilliQ||Total||Incubation | ||
| Line 202: | Line 202: | ||
====Monday, July 26 <span class="by">By: Wataru, Tomonori, Makoto</span>==== | ====Monday, July 26 <span class="by">By: Wataru, Tomonori, Makoto</span>==== | ||
| - | ===== | + | =====Electrophoresis of PCR Products===== |
[[Image:KyotoExp100726-1.png|300px|right]] | [[Image:KyotoExp100726-1.png|300px|right]] | ||
{| class="electrophoresis" | {| class="electrophoresis" | ||
| Line 231: | Line 231: | ||
|6||SRRZ||59.6||SRRz<sub>Sam7</sub>(2) | |6||SRRZ||59.6||SRRz<sub>Sam7</sub>(2) | ||
|} | |} | ||
| - | ===== | + | =====Transformation===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Well||Sample||Competent Cell||Total||Plate||Incubation||Colony | !Name||Well||Sample||Competent Cell||Total||Plate||Incubation||Colony | ||
| Line 245: | Line 245: | ||
====Tuesday, July 27 <span class="by">By: Wataru, Tomo, Kazuya, Ken, Naoi==== | ====Tuesday, July 27 <span class="by">By: Wataru, Tomo, Kazuya, Ken, Naoi==== | ||
| - | ===== | + | =====Colony PCR of S<sub>Sam7</sub>-E0840 (Electrophoresis for 35min)===== |
[[Image:KyotoExp100727-1.png|300px|right]] | [[Image:KyotoExp100727-1.png|300px|right]] | ||
{| class="electrophoresis" | {| class="electrophoresis" | ||
| Line 281: | Line 281: | ||
|} | |} | ||
Marker: 1kb, 100bp | Marker: 1kb, 100bp | ||
| - | ===== | + | =====Miniprep===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Concentration | !Name||Concentration | ||
| Line 291: | Line 291: | ||
|E0840||120.1 | |E0840||120.1 | ||
|} | |} | ||
| - | ===== | + | =====Restriction Digestion===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Sample||2 buffer||BSA||colspan="2"|Enzyme 1||colspan="2"|Enzyme 2||MilliQ||Total||Incubation | !Name||Sample||2 buffer||BSA||colspan="2"|Enzyme 1||colspan="2"|Enzyme 2||MilliQ||Total||Incubation | ||
| Line 301: | Line 301: | ||
|SRRz<sub>Sam7</sub>(2)||40||5||0.5||EcoRI||0.4||SpeI||0.4||3.8||50 | |SRRz<sub>Sam7</sub>(2)||40||5||0.5||EcoRI||0.4||SpeI||0.4||3.8||50 | ||
|} | |} | ||
| - | ===== | + | =====Ligation===== |
| - | ===== | + | =====Transformation===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Sample||Competent Cells||Total||Plate||Incubation||Colony | !Name||Sample||Competent Cells||Total||Plate||Incubation||Colony | ||
| Line 313: | Line 313: | ||
====Wednesday, July 28 <span class="by">By: </span>==== | ====Wednesday, July 28 <span class="by">By: </span>==== | ||
| - | ===== | + | =====Miniprep===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Concentration | !Name||Concentration | ||
| Line 322: | Line 322: | ||
|} | |} | ||
Diluted S<sub>Sam7</sub>(1)-E0840 and S<sub>Sam7</sub>(2)-E0840 20 times with water, and used as template DNA. | Diluted S<sub>Sam7</sub>(1)-E0840 and S<sub>Sam7</sub>(2)-E0840 20 times with water, and used as template DNA. | ||
| - | ===== | + | =====Deletion PCR to delete a functional domain of S gene===== |
{| class="experiments" | {| class="experiments" | ||
!||Water||MgSO4||dNTPs||10xBuffer||Primer Fwd.||Primer Rev.||Template (1)||Template (2)||KOD Plus ver.2||Total | !||Water||MgSO4||dNTPs||10xBuffer||Primer Fwd.||Primer Rev.||Template (1)||Template (2)||KOD Plus ver.2||Total | ||
| Line 346: | Line 346: | ||
|} | |} | ||
| - | ===== | + | =====Restriction Digestion to check the function of DpnI===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Sample||fast digestion buffer||DpnI||MilliQ||Total | !Name||Sample||fast digestion buffer||DpnI||MilliQ||Total | ||
| Line 354: | Line 354: | ||
|S<sub>Sam7</sub>(2)-E0840||3||1||0.1||5.8||10 | |S<sub>Sam7</sub>(2)-E0840||3||1||0.1||5.8||10 | ||
|} | |} | ||
| - | ===== | + | =====Electrophoresis for 35min===== |
| - | [[ | + | [[Image:KyotoExp100728-1.png|300px|right]] |
{| class="electrophoresis" | {| class="electrophoresis" | ||
!No.||Name||Length||Result | !No.||Name||Length||Result | ||
| Line 372: | Line 372: | ||
====Thursday, July 29 <span class="by">By: </span>==== | ====Thursday, July 29 <span class="by">By: </span>==== | ||
| - | ===== | + | =====Restriction Digestion===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Sample volume||Fastdigestion Buffer||colspan="2"|Enzyme 1||MilliQ||Total||Incubation | !Name||Sample volume||Fastdigestion Buffer||colspan="2"|Enzyme 1||MilliQ||Total||Incubation | ||
| Line 380: | Line 380: | ||
|S<sub>Sam7,ΔTMD1</sub>(2)-E0840 (1)||50||6||DpnI||0.2||3.8||60 | |S<sub>Sam7,ΔTMD1</sub>(2)-E0840 (1)||50||6||DpnI||0.2||3.8||60 | ||
|} | |} | ||
| - | ===== | + | =====Ligation and Phosphorylation===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Sample||MilliQ||Ligation High||T4 Kinase||Total||Incubation | !Name||Sample||MilliQ||Ligation High||T4 Kinase||Total||Incubation | ||
| Line 388: | Line 388: | ||
|S<sub>Sam7,ΔTMD1</sub>(2)-E0840 (1)||2||7||5||1||15 | |S<sub>Sam7,ΔTMD1</sub>(2)-E0840 (1)||2||7||5||1||15 | ||
|} | |} | ||
| - | ===== | + | =====Transformation===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Sample Volume||Competent Cell||Total||Plate||Incubation||Colony | !Name||Sample Volume||Competent Cell||Total||Plate||Incubation||Colony | ||
| Line 399: | Line 399: | ||
====Monday, August 2 <span class="by">By: Wataru, Ken</span>==== | ====Monday, August 2 <span class="by">By: Wataru, Ken</span>==== | ||
| - | ===== | + | =====Miniprep===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Concentration | !Name||Concentration | ||
| Line 413: | Line 413: | ||
|R0011||18.6 | |R0011||18.6 | ||
|} | |} | ||
| - | ===== | + | =====PCR of E0240===== |
E0240 is very important parts to measure RPU of promoters in iGEM. However, we failed to transfect it to E.coli from parts kit of iGEM. So we decided to amplify this parts by PCR. | E0240 is very important parts to measure RPU of promoters in iGEM. However, we failed to transfect it to E.coli from parts kit of iGEM. So we decided to amplify this parts by PCR. | ||
{| class="experiments" | {| class="experiments" | ||
| Line 433: | Line 433: | ||
|4℃||forever|| | |4℃||forever|| | ||
|} | |} | ||
| - | ===== | + | =====Electrophoresis===== |
| - | ===== | + | =====PCR Purification===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Concentration | !Name||Concentration | ||
| Line 442: | Line 442: | ||
|E0240(2)||55.3 | |E0240(2)||55.3 | ||
|} | |} | ||
| - | ===== | + | =====Restriction Digestion for inserting E0240 to pSB4K5 by 3A assembly===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Sample volume||2 buffer||BSA||colspan="2"|Enzyme 1||colspan="2"|Enzyme 2||MilliQ||Total | !Name||Sample volume||2 buffer||BSA||colspan="2"|Enzyme 1||colspan="2"|Enzyme 2||MilliQ||Total | ||
| Line 478: | Line 478: | ||
|4℃||forever|| | |4℃||forever|| | ||
|} | |} | ||
| - | ===== | + | =====Restriction Digestion of S<sub>Sam7,ΔTMD1</sub>-E0840 by DpnI===== |
| - | ===== | + | =====Transformation===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Sample||Competent Cells||Total||Plate||Incubation||Colony | !Name||Sample||Competent Cells||Total||Plate||Incubation||Colony | ||
| Line 493: | Line 493: | ||
=====Culture===== | =====Culture===== | ||
Picked two colonies from S<sub>Sam7,ΔTMD1</sub>-E0840 (1), and S<sub>Sam7,ΔTMD1</sub>-E0840 (3), and cultured at 37℃ from 08/03 to 08/04. | Picked two colonies from S<sub>Sam7,ΔTMD1</sub>-E0840 (1), and S<sub>Sam7,ΔTMD1</sub>-E0840 (3), and cultured at 37℃ from 08/03 to 08/04. | ||
| - | ===== | + | =====Miniprep===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Concentration | !Name||Concentration | ||
| Line 501: | Line 501: | ||
|R0011||26.8 | |R0011||26.8 | ||
|} | |} | ||
| - | ===== | + | =====Restriction Digestion===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Sample||2 buffer||BSA||colspan="2"|Enzyme 1||colspan="2"|Enzyme 2||MilliQ||Total | !Name||Sample||2 buffer||BSA||colspan="2"|Enzyme 1||colspan="2"|Enzyme 2||MilliQ||Total | ||
| Line 526: | Line 526: | ||
=====Ethanol Precipitation===== | =====Ethanol Precipitation===== | ||
After ethanol precipitation, we diluted pSB4K5 by 2µL MilliQ | After ethanol precipitation, we diluted pSB4K5 by 2µL MilliQ | ||
| - | ===== | + | =====Ligation===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Vector||colspan="2"|Insert 1||colspan="2"|Insert 2||Ligation High||Total||Incubation | !Name||Vector||colspan="2"|Insert 1||colspan="2"|Insert 2||Ligation High||Total||Incubation | ||
| Line 534: | Line 534: | ||
|R0011-E0240(2) [pSB4K5]||pSB4K5 [EP]||1||R0011 [ES]||1||E0240(2) [XP]||1||3||15 | |R0011-E0240(2) [pSB4K5]||pSB4K5 [EP]||1||R0011 [ES]||1||E0240(2) [XP]||1||3||15 | ||
|} | |} | ||
| - | ===== | + | =====PCR of I20260===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Water||MgSO4||dNTPs||10xBuffer||Primer VF2||Primer VR||Template I20260||KOD plus ver.2||Total | !Name||Water||MgSO4||dNTPs||10xBuffer||Primer VF2||Primer VR||Template I20260||KOD plus ver.2||Total | ||
| Line 553: | Line 553: | ||
|4℃||forever|| | |4℃||forever|| | ||
|} | |} | ||
| - | ===== | + | =====PCR Purification===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Concentration | !Name||Concentration | ||
| Line 559: | Line 559: | ||
|I20260||40.6 ng/µL | |I20260||40.6 ng/µL | ||
|} | |} | ||
| - | ===== | + | =====Restriction Digestion===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Sample volume||2 buffer||BSA||colspan="2"|Enzyme 1||colspan="2"|Enzyme 2||MilliQ||Total | !Name||Sample volume||2 buffer||BSA||colspan="2"|Enzyme 1||colspan="2"|Enzyme 2||MilliQ||Total | ||
| Line 565: | Line 565: | ||
|I20260 [EP]||45 µL||6||0.6||EcoRI||0.2||PstI||0.2||8||60 | |I20260 [EP]||45 µL||6||0.6||EcoRI||0.2||PstI||0.2||8||60 | ||
|} | |} | ||
| - | ===== | + | =====PCR Purification===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Concentration||Volume | !Name||Concentration||Volume | ||
| Line 572: | Line 572: | ||
|} | |} | ||
I20260 [EP] is concentrated at 7µL | I20260 [EP] is concentrated at 7µL | ||
| - | ===== | + | =====Ligation===== |
{| class="experiments" | {| class="experiments" | ||
!||Vector||colspan="2"|Insert||colspan="2"|Ligation High||Total||Incubation | !||Vector||colspan="2"|Insert||colspan="2"|Ligation High||Total||Incubation | ||
| Line 721: | Line 721: | ||
|13||I20260 [pSB4K5] (2) [EP]||960, 4339||○ | |13||I20260 [pSB4K5] (2) [EP]||960, 4339||○ | ||
|} | |} | ||
| - | [[Image:KyotoExp100806-1.png]] | + | [[Image:KyotoExp100806-1.png|300px|right]] |
White colonies are not inserted R0011 but its vector. Top10 we used are deleted Lac operon. Then, correctly inserted parts is green because of the lack of ''lac''I gene. | White colonies are not inserted R0011 but its vector. Top10 we used are deleted Lac operon. Then, correctly inserted parts is green because of the lack of ''lac''I gene. | ||
=====Error PCR (Retry)===== | =====Error PCR (Retry)===== | ||
| Line 885: | Line 885: | ||
|8||R0011 [pSB4K5, C2] (2-N)|||| | |8||R0011 [pSB4K5, C2] (2-N)|||| | ||
|} | |} | ||
| - | [[image:KyotoExp100811-1.png]] | + | [[image:KyotoExp100811-1.png|300px|right]] |
Each enzyme correctly cut samples. | Each enzyme correctly cut samples. | ||
=====Screening PCR of SRRz===== | =====Screening PCR of SRRz===== | ||
| Line 902: | Line 902: | ||
|} | |} | ||
Marker: Lambda Marker | Marker: Lambda Marker | ||
| - | [[image:KyotoExp100811-2.png]] | + | [[image:KyotoExp100811-2.png|300px|right]] |
| - | [[image:KyotoExp100811-3.png]] | + | [[image:KyotoExp100811-3.png|300px|right]] |
Discussion: All of the sample were self-ligation of DT. SRRz weren't inserted. | Discussion: All of the sample were self-ligation of DT. SRRz weren't inserted. | ||
| Line 927: | Line 927: | ||
|} | |} | ||
Maker: Lambda, 100bp | Maker: Lambda, 100bp | ||
| - | [[image:KyotoExp100812-1.png]] | + | [[image:KyotoExp100812-1.png|300px|right]] |
Discussion: Each enzyme correctly cut each sample and was active. | Discussion: Each enzyme correctly cut each sample and was active. | ||
| Line 971: | Line 971: | ||
|} | |} | ||
Marker: Lambda, 100bp | Marker: Lambda, 100bp | ||
| - | [[Image:KyotoExp100819-1.png]] | + | [[Image:KyotoExp100819-1.png|300px|right]] |
=====Ligation and Transformation===== | =====Ligation and Transformation===== | ||
{| class="experiments | {| class="experiments | ||
| Line 986: | Line 986: | ||
====Friday, August 20 <span class="by">By: Wataru, Ken</span>==== | ====Friday, August 20 <span class="by">By: Wataru, Ken</span>==== | ||
=====Making Culture and Master Plate of S<sub>ΔTMD1</sub>-E0840===== | =====Making Culture and Master Plate of S<sub>ΔTMD1</sub>-E0840===== | ||
| - | ===== | + | =====Miniprep===== |
{| class="expeirments" | {| class="expeirments" | ||
!Name||Concentration | !Name||Concentration | ||
| Line 992: | Line 992: | ||
|B0015||41.1 ng/µL | |B0015||41.1 ng/µL | ||
|} | |} | ||
| - | ===== | + | =====PCR of SRRz===== |
{| class="experiments" | {| class="experiments" | ||
!Name||10xBuffer||MgS04||dNTP||Template||colspan="2"|Primer Fwd.||Primer Rev.||MilliQ||KOD plus ver.2||Total | !Name||10xBuffer||MgS04||dNTP||Template||colspan="2"|Primer Fwd.||Primer Rev.||MilliQ||KOD plus ver.2||Total | ||
|- | |- | ||
| - | |SRRz (1)||5 µL||3||5||5||F1||1.5||1.5||28||1||50 | + | |SRRz<sub>Sam7</sub> (1)||5 µL||3||5||5||F1||1.5||1.5||28||1||50 |
|- | |- | ||
| - | |SRRz (2)||5||3||5||5||F2||1.5||1.5||28||1||50 | + | |SRRz<sub>Sam7</sub> (2)||5||3||5||5||F2||1.5||1.5||28||1||50 |
|- | |- | ||
| - | |SRRz (3)||5||3||5||5||F1||1.5||1.5||28||1||50 | + | |SRRz<sub>Sam7</sub> (3)||5||3||5||5||F1||1.5||1.5||28||1||50 |
|- | |- | ||
| - | |SRRz (4)||5||3||5||5||F2||1.5||1.5||28||1||50 | + | |SRRz<sub>Sam7</sub> (4)||5||3||5||5||F2||1.5||1.5||28||1||50 |
|- | |- | ||
| - | |SRRz (5)||5||3||5||5||F1||1.5||1.5||28||1||50 | + | |SRRz<sub>Sam7</sub> (5)||5||3||5||5||F1||1.5||1.5||28||1||50 |
|- | |- | ||
| - | |SRRz (6)||5||3||5||5||F2||1.5||1.5||28||1||50 | + | |SRRz<sub>Sam7</sub> (6)||5||3||5||5||F2||1.5||1.5||28||1||50 |
|} | |} | ||
{| class="experiments" | {| class="experiments" | ||
| Line 1,019: | Line 1,019: | ||
|4℃||forever|| | |4℃||forever|| | ||
|} | |} | ||
| - | ===== | + | =====Electrophoresis===== |
{| class="electrophoresis" | {| class="electrophoresis" | ||
!Name | !Name | ||
|- | |- | ||
| - | |SRRz (1) | + | |SRRz<sub>Sam7</sub> (1) |
|- | |- | ||
| - | |SRRz (3) | + | |SRRz<sub>Sam7</sub> (3) |
|- | |- | ||
| - | |SRRz (5) | + | |SRRz <sub>Sam7</sub>(5) |
|- | |- | ||
| - | |SRRz (2) | + | |SRRz<sub>Sam7</sub> (2) |
|- | |- | ||
| - | |SRRz (4) | + | |SRRz<sub>Sam7</sub> (4) |
|- | |- | ||
| - | |SRRz (6) | + | |SRRz<sub>Sam7</sub> (6) |
|} | |} | ||
| - | [[Image:KyotoExp100820-1.png]] | + | [[Image:KyotoExp100820-1.png|300px|right]] |
Discussion: Primer F1 might be better than F2, because the bands of 1, 2 and 3 were clearer. We decided to use sample 1 and 3. Their bands were clearer in the three. | Discussion: Primer F1 might be better than F2, because the bands of 1, 2 and 3 were clearer. We decided to use sample 1 and 3. Their bands were clearer in the three. | ||
| - | ===== | + | =====PCR Purification===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Concentration | !Name||Concentration | ||
|- | |- | ||
| - | |SRRz (1)||134.0 ng/µL | + | |SRRz<sub>Sam7</sub> (1)||134.0 ng/µL |
|- | |- | ||
| - | |SRRz (3)||69.0 | + | |SRRz<sub>Sam7</sub> (3)||69.0 |
|} | |} | ||
| - | ===== | + | =====Restriction Digestion===== |
{| class="experiments" | {| class="experiments" | ||
!Name||Sample||10xBuffer||100xBuffer||EcoRI||XbaI||SpeI||MilliQ||Total||Incubation | !Name||Sample||10xBuffer||100xBuffer||EcoRI||XbaI||SpeI||MilliQ||Total||Incubation | ||
| Line 1,051: | Line 1,051: | ||
|B0015 [EX]||50 µL||6||0.6||0.4||0.4||-||2.6||60||rowspan="3"|17:45-18:45 | |B0015 [EX]||50 µL||6||0.6||0.4||0.4||-||2.6||60||rowspan="3"|17:45-18:45 | ||
|- | |- | ||
| - | |SRRz (1) [EP]||50||6||0.6||0.4||-||0.4||2.6||60 | + | |SRRz<sub>Sam7</sub> (1) [EP]||50||6||0.6||0.4||-||0.4||2.6||60 |
|- | |- | ||
| - | |SRRz (3) [EP]||50||6||0.6||0.4||-||0.4||2.6||60 | + | |SRRz<sub>Sam7</sub> (3) [EP]||50||6||0.6||0.4||-||0.4||2.6||60 |
|} | |} | ||
=====Purification===== | =====Purification===== | ||
| Line 1,059: | Line 1,059: | ||
!Name||Concentration | !Name||Concentration | ||
|- | |- | ||
| - | |SRRz (1) [EP]||109.0 ng/µL | + | |SRRz<sub>Sam7</sub> (1) [EP]||109.0 ng/µL |
|- | |- | ||
| - | |SRRz (2) [EP]||110.0 | + | |SRRz<sub>Sam7</sub> (2) [EP]||110.0 |
|- | |- | ||
|B0015||25.5 | |B0015||25.5 | ||
|} | |} | ||
| - | =====[[ | + | =====Ligation and Transformation===== |
| + | ---- | ||
| + | |||
| + | ====Monday, August 23 <span class="by">By: Wataru, Tomo, Ken, Fumitaka, Tasuku</span>==== | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | |Sample number||Concentration | ||
| + | |- | ||
| + | |S<sub>ΔTMD1</sub>-E0840 (1-1)||58.9 ng/µL | ||
| + | |- | ||
| + | |S<sub>ΔTMD1</sub>-E0840 (2-2)||49.9 | ||
| + | |} | ||
| + | =====Sequence===== | ||
| + | Sample: S<sub>ΔTMD1</sub>-E0840 (1-1). S<sub>ΔTMD1</sub>-E0840 (2-2), I20260 [pSB4K5] | ||
| + | Discussion: The sequencing was in success and the results were desirable. It meant the point mutation was succeeded and sequence of I20260 [pSB4K5] was confirmed. We decided to use S<sub>ΔTMD1</sub>-E0840. | ||
| + | =====Screening PCR of SRRz<sub>Sam7</sub>-B0015===== | ||
| + | {| class="experiments" | ||
| + | |90℃||10min|| | ||
| + | |- | ||
| + | |94℃||30s||rowspan="3"|35cycles | ||
| + | |- | ||
| + | |50℃||30s | ||
| + | |- | ||
| + | |72℃||1.5min | ||
| + | |- | ||
| + | |72℃||4min|| | ||
| + | |- | ||
| + | |4℃||hold|| | ||
| + | |} | ||
| + | |||
| + | {| class="electrophoresis" | ||
| + | !No.||Name | ||
| + | |- | ||
| + | |1-13||SRRz<sub>Sam7</sub>-B0015 | ||
| + | |- | ||
| + | |C||Control: B0015 | ||
| + | |- | ||
| + | |N||None | ||
| + | |} | ||
| + | Marker: Lambda, 100bp | ||
| + | [[Image:KyotoExp100823-1.png|300px|right]] | ||
| + | Discussion: We found the band; about 200bp, and it meant the lligation was completed successfully. | ||
| + | =====Deletion PCR of S<sub>ΔTMD1</sub>-E0840 (2-2)===== | ||
| + | {| class="experiments" | ||
| + | !Name||Sample||10xBuffer||dNTPs||Primer Fwd.||Primer Rev.||Template||Water||KOD-plus-||Total | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (1)||5 µL||5||1.5||1.5||1||35||1||50 | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (2)||5||5||1.5||1.5||1||35||1||50 | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (Control)||5||5||1.5||1.5||1||35||-||50 | ||
| + | |} | ||
| + | {| class="experiments" | ||
| + | |94℃||2min|| | ||
| + | |- | ||
| + | |94℃||10s||rowspan="3"|35cycles | ||
| + | |- | ||
| + | |56℃||30s | ||
| + | |- | ||
| + | |68℃||3.5min | ||
| + | |- | ||
| + | |4℃||forever|| | ||
| + | |} | ||
| + | =====Restriction Digestion (DpnI)===== | ||
| + | {| class="experiments" | ||
| + | !Sample||DpnI||Total||Incubation | ||
| + | |- | ||
| + | |25 µL||1||26||19:00-20:10 | ||
| + | |} | ||
| + | =====Ligation===== | ||
| + | {| class="experiments" | ||
| + | !Name||Sample||Water||Ligation high||T4 Kinase||Total||Incubation | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (1)||3 µL||6||5||1||15||20:15-21:15 | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (2)||3||6||5||1||15||20:15-21:15 | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (Control)||3||6||5||1||15 | ||
| + | |} | ||
| + | =====Transformation===== | ||
| + | |||
| + | |||
| + | ====Tuesday, August 24 <span class="by">By: Ken, Tomo, Tasuku, Takuya</span>==== | ||
| + | =====Retry of deletion PCR of S<sub>ΔTMD1</sub>-E0840===== | ||
| + | {| class="experiments" | ||
| + | !Name||Sample||10xBuffer||dNTPs||MgSO4||Primer1||Primer2||Template||Water||KOD-plus-||Total | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (1)||5 µL||5||3||1.5||1.5||1||32||1||50 | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (2)||5||5||3||1.5||1.5||1||32||1||50 | ||
| + | |- | ||
| + | |Control||5||5||3||1.5||1.5||1||32||1||50 | ||
| + | |} | ||
| + | {| class="experiments" | ||
| + | |94℃||2min|| | ||
| + | |- | ||
| + | |94℃||10s||rowspan="3"|35cycles | ||
| + | |- | ||
| + | |58℃||30s | ||
| + | |- | ||
| + | |68℃||3.5min | ||
| + | |- | ||
| + | |4℃||hold|| | ||
| + | |} | ||
| + | =====Restriction Digestion (DpnI)===== | ||
| + | 14:15-15:15 | ||
| + | =====Electrophoreis===== | ||
| + | {| class="electrophoresis" | ||
| + | !Lane||Name | ||
| + | |- | ||
| + | |1||rrS<sub>ΔTMD1</sub>-E0840 (1) | ||
| + | |- | ||
| + | |2||rrS<sub>ΔTMD1</sub>-E0840 (3) | ||
| + | |- | ||
| + | |C||rrS<sub>ΔTMD1</sub>-E0840 (Control) | ||
| + | |} | ||
| + | Marker: 100bp, Lambda. | ||
| + | [[Image:KyotoExp100824-1.png|300px|right]] | ||
| + | We found the band of sample 1 and 2 about 3000bp and there wasn't the band of sample control. So, we confirmed the PCR and Restriction Digestion were completed successfully. | ||
| + | =====Ligation===== | ||
| + | =====Point mutation of SRRz===== | ||
| + | {| class="experiments" | ||
| + | !Name||10x||dNTPs||MgSO4||Primer1||Primer2||Template||Water||KOD-plus-||total | ||
| + | |- | ||
| + | |SRRz<sub>Sam7</sub>-B0015 (1)||5||5||3||1.5||1.5||1||32||1||50 | ||
| + | |- | ||
| + | |SRRz<sub>Sam7</sub>-B0015 (2)||5||5||3||1.5||1.5||1||32||1||50 | ||
| + | |- | ||
| + | |SRRz<sub>Sam7</sub>-B0015 (Control)||5||5||3||1.5||1.5||1||32||1||50 | ||
| + | |} | ||
| + | {| class="experiments" | ||
| + | |94℃||2min|| | ||
| + | |- | ||
| + | |98℃||10s||rowspan="3"|30cycles | ||
| + | |- | ||
| + | |55℃||30s | ||
| + | |- | ||
| + | |68℃||4min | ||
| + | |- | ||
| + | |4℃||hold|| | ||
| + | |} | ||
| + | =====Restriction Digestion (DpnI), Electrophoresis and Ligation===== | ||
| + | [[Image:KyotoExp100824-2.png|300px|right]] | ||
| + | We could find point mutation PCR and restriction enzyme of DpnI was done. | ||
| + | ====PCR of E0240==== | ||
| + | {| class="experiments" | ||
| + | !Sample||10xBuffer||dNTPs||MgSO4||VF2||VR||Template||Water||KOD-plus-||Total | ||
| + | |- | ||
| + | |E0240 (1)||5||5||3||1.5||1.5||1||31.5||1||50 | ||
| + | |- | ||
| + | |E0240 (2)||5||5||3||1.5||1.5||1||31.5||1||50 | ||
| + | |- | ||
| + | |} | ||
| + | =====PCR Purification===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |E0240 (1)||5.5 x 50 ng/µL | ||
| + | |- | ||
| + | |E0240 (2)||5.2 x 50 | ||
| + | |} | ||
| + | =====Restriction Digestion (EcoRI, PstI) and Gel Extraction===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |E0240 (1)|| 28.8 ng/µL | ||
| + | |- | ||
| + | |E0240 (2)|| 26.4 | ||
| + | |} | ||
| + | =====Transformation===== | ||
| + | |||
| + | |||
| + | ====Wednesday, August 25 <span class="by">By:Ken, Tomo, Kazuya, Tasuku, Takuya<span>==== | ||
| + | =====Making culture and Master plate===== | ||
| + | {| class="experiments" | ||
| + | !Name||Colony | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (1)||○ | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (2)||○ | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (Control)||} | ||
| + | |- | ||
| + | |SRRz<sub>Sam7</sub>-B0015 (1)||○ | ||
| + | |- | ||
| + | |SRRz<sub>Sam7</sub>-B0015 (2)||○ | ||
| + | |- | ||
| + | |SRRz<sub>Sam7</sub>-B0015 (Control)||} | ||
| + | |} | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |pSB4K5||29.0 ng/µL | ||
| + | |} | ||
| + | =====Restriction Digestion===== | ||
| + | {| class="experiments" | ||
| + | !Sample name||Template||10xbuffer||100xbuffer||EcoRI||SpeI||PstI||Water||Total | ||
| + | |- | ||
| + | |pSB4K5||50||6||0.6||0.4||0.4||-||2.6||60 | ||
| + | |- | ||
| + | |R0011 [pSB4K5]||10||4||0.4||-||0.3||0.3||25||40 | ||
| + | |} | ||
| + | =====Purification===== | ||
| + | {| class="experiments" | ||
| + | |Sample Name||Concentration | ||
| + | |- | ||
| + | |pSB4K5||18.4 ng/µL | ||
| + | |- | ||
| + | |R0011 [pSB4K5]||8.6 | ||
| + | |} | ||
| + | =====Ligation of E0240 and pSB4K5, Transformation===== | ||
| + | |||
| + | |||
| + | ====Thursday, August 26 <span class="by">By:Ken, Tomo, Kazuya, Tasuku, Takuya, Fumitaka</span> | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | |Sample name||Concentration | ||
| + | |- | ||
| + | |J23116 (RPU0.7)||44.5 ng/µL | ||
| + | |} | ||
| + | =====Restriction Digestion===== | ||
| + | {| class="experiments" | ||
| + | !Name||Template||10xbuffer||100xbuffer||SpeI||PstI||Water||Total | ||
| + | |- | ||
| + | |J23116 (RPU0.7)||25||4||0.4||0.3||0.3||10||40 | ||
| + | |} | ||
| + | =====Purification of===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |J23116 (RPU0.7)||49.8 ng/µL | ||
| + | |} | ||
| + | |||
| + | |||
| + | ====Friday, August 27 <span class="by">By: Ken, Tomo, Kazuya, Fumitaka</span>==== | ||
| + | =====Making master plate of E0240 [pSB4K5]===== | ||
| + | {| class="experiments" | ||
| + | |Sample Name||Concentration | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (1-2)||20.9 ng/µL | ||
| + | |- | ||
| + | |SRRz-B0015 (1-1)||16.4 | ||
| + | |} | ||
| + | =====Restriction Digestion===== | ||
| + | {| class="experiments" | ||
| + | !Name||Template||10xbuffer||100xbuffer||XbaI||PstI||Water||Total||Incubation | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (1-2)||45 µL||6||0.6||0.3||0.3||7.8||60||rowspan="2"|13:20-14:20 | ||
| + | |- | ||
| + | |SRRz-B0015 (1-1)||45||6||0.6||0.3||0.3||7.8||60 | ||
| + | |} | ||
| + | |||
| + | =====Purification===== | ||
| + | {|Sample Name||Concentration | ||
| + | |- | ||
| + | |rrS<sub>ΔTMD1</sub>-E0840 (1-2)||44.7 ng/µL | ||
| + | |- | ||
| + | |SRRz-B0015 (1-1)||56.1 | ||
| + | |} | ||
| + | =====Ligation and Transformation===== | ||
| + | {| class="experiments" | ||
| + | !Name | ||
| + | |- | ||
| + | |R0011-rrS<sub>ΔTMD1</sub>-E0840 (1-2) | ||
| + | |- | ||
| + | |J23116 (RPU0.7)- rrS<sub>ΔTMD1</sub>-E0840 (1-2) | ||
| + | |- | ||
| + | |R0011-SRRz-B0015 (1-1) [pSB4K5] | ||
| + | |} | ||
| + | |||
| + | ====Monday, August 30 <span class="by">By: Tomonori, Kazuya, Tasuku, Ken</span>==== | ||
| + | =====Making culture and Master plate===== | ||
| + | {| class="experiments" | ||
| + | !Name||Colony | ||
| + | |R0011-rrS<sub>ΔTMD1</sub>-E0840||Many colonies | ||
| + | |- | ||
| + | |R0011-rrS<sub>ΔTMD1</sub>-E0840 (Control)||Some colonies | ||
| + | |- | ||
| + | |J23116 (RPU0.7)- rrS<sub>ΔTMD1</sub>-E0840||Many colonies | ||
| + | |- | ||
| + | |J23116 (RPU0.7)- rrS<sub>ΔTMD1</sub>-E0840 (Control)||Many colonies | ||
| + | |- | ||
| + | |R0011-SRRz-B0015 (1-1) [pSB4K5]||No colony | ||
| + | |- | ||
| + | |R0011-SRRz-B0015 (1-1) [pSB4K5] (Control)||No colony | ||
| + | |} | ||
| + | Discussion: There ware some colonies, which emitted green light, on the plate 1. So, we cultured those colonies on master plate. | ||
| + | On the plate 5 and 6, even though we used KRX, which is able to repress lac promoter, colonies might be dead. However, we still have to do some experience so that we confirm lac promoter cannot repress enough and E. coli cannot survive. | ||
| + | |||
| + | |||
| + | ====Tuesday, August 31 <span class="by">By: Tomonori, Takuya Y., Kazuya, Tasuku, Takuya, Ken<span> | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |- | ||
| + | |J23105 (RPU0.3)||48.5 ng/µL | ||
| + | |- | ||
| + | |R0011-rrS<sub>ΔTMD1</sub>-E0840||107.3 | ||
| + | |} | ||
| + | =====Restriction Digestion===== | ||
| + | =====Gel Extraction of R0011-rrS<sub>ΔTMD1</sub>-E0840 (Electrophoresis for 45min)===== | ||
| + | <!--[[image:KyotoExp100831-1.png|300px|right]]--> | ||
| + | Discussion: There were two band at the bottom of the gel. It was too long -45min-, and insert and vector might be contaminated. But we went on next operation. | ||
| + | =====Purification of J23105 (RPU0.3) and R0011-rrS<sub>ΔTMD1</sub>-E0840===== | ||
| + | {| class="experiments" | ||
| + | !Name||Concentration | ||
| + | |J23105 (RPU0.3)||5.8 ng/µL | ||
| + | |- | ||
| + | |R0011-rrS<sub>ΔTMD1</sub>-E0840||7.8 | ||
| + | |} | ||
| + | =====Ligation and Transformation===== | ||
| + | {| class="experiments" | ||
| + | |Insert||Vector | ||
| + | |- | ||
| + | |R0011-rrS<sub>ΔTMD1</sub>-E0840||J23105 (RPU0.3) | ||
| + | |} | ||
| + | |||
| + | |||
| + | ====Wednesday, September 1 <span class="by">By: Tomonori, Kazuya, Tasuku, Fumitaka, Ken</span>==== | ||
| + | =====Making culture and Master plate===== | ||
| + | {| class="experiments" | ||
| + | !Name||Colony | ||
| + | |- | ||
| + | |R0011-rrS<sub>ΔTMD1</sub>-E0840-J23105 (RPU0.3)||Many colonies | ||
| + | |- | ||
| + | |R0011-rrS<sub>ΔTMD1</sub>-E0840-J23105 (RPU0.3) (Control)||Many colonies | ||
| + | |} | ||
| + | =====Screenig PCR of R0011-rrS<sub>ΔTMD1</sub>-E0840-J23105 (RPU0.3)===== | ||
| + | * Sample: 1-13 | ||
| + | * Control: Positive (B0015) | ||
| + | * Maker: lambda, 100 | ||
| + | <!--M, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, P, M | ||
| + | [[image:KyotoExp100901-1.png|300px|right]]--> | ||
| + | Discussion: All of the sample except sample 10 might be self-ligation products of J23105 (RPU0.3). | ||
| + | =====Miniprep===== | ||
| + | {| class="experiments" | ||
| + | |SRRz-B0015 (1-1)||33.8 ng/µL | ||
| + | |- | ||
| + | |pSB4K5||56.0 | ||
| + | |} | ||
| + | =====Restriction Digestion of SRRz and pSB4K5===== | ||
| + | {| class="experiments" | ||
| + | !Name||Template||10xbuffer||100xbuffer||EcoRI||PstI||Water||Total||Incubation | ||
| + | |- | ||
| + | |SRRz-B0015 (1-1)||20 µL||4||0.4||0.3||0.3||15||40||rowspan="2"|13:25-14:30 | ||
| + | |- | ||
| + | |pSB4K5||20||4||0.4||0.3||0.3||15||40 | ||
| + | |} | ||
| + | =====Purification===== | ||
| + | {| class="experiments" | ||
| + | |SRRz-B0015 (1-1)||6.5 ng/µL | ||
| + | |- | ||
| + | |pSB4K5||16.8 | ||
| + | |} | ||
| + | =====Ligation and transformation==== | ||
| + | * Insert: SRRz-B0015 (1-1) | ||
| + | * Vector: pSB4K5 | ||
| + | |||
| + | |||
| + | ====Thursday, September 2 <span class="by">By: Tomonori, Tomo, Takuya, Ken<span>==== | ||
| + | =====Making culture and Master plate===== | ||
| + | {| class="experiments" | ||
| + | |SRRz-B0015 (1-1) [pSB4K5]||13 colonies | ||
| + | |- | ||
| + | |SRRz-B0015 (1-1) [pSB4K5] (Control)||13 colonies | ||
| + | |} | ||
| + | =====Screening PCR of rSRRz low===== | ||
| + | Sample: (1-13) SRRz-B0015 (1-1) [pSB4K5] | ||
| + | Maker: Lambda, 100 | ||
| + | Control: Positive (B0015), Neganive | ||
| + | <!--M, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, P, N, M | ||
| + | [[image:KyotoExp100902-1.png|300px|right]]--> | ||
| + | Discussion: From sample 1, two vectors might be ligated. Sample 3 and 4, SRRz-B0015 might be inserted in low copy plasmid correctly. Sample 11, it might be the self-ligation product of low copy plasmid. Anyway, we decided to culture those 4 colonies on master plate. | ||
| + | |||
| + | |||
| + | ====Friday, September 3 <span class="by">By: Tomonori, Tomo, Kazuya, Tasuku, Fumitaka, Ken</span>==== | ||
| + | =====Making culture===== | ||
| + | *R0011-rrS<sub>ΔTMD1</sub>-E0840 (1) | ||
| + | *R0011-rrS<sub>ΔTMD1</sub>-E0840 (3) | ||
| + | *rrS<sub>ΔTMD1</sub>-E0840 (1-1) | ||
| + | *rrS<sub>ΔTMD1</sub>-E0840 (1-2) | ||
| + | *SRRz-B0015 (1-1) [pSB4K5] | ||
| + | *SRRz-B0015 (1-2) [pSB4K5] | ||
| + | *R0011-E0240 [pSB4K5] | ||
---- | ---- | ||
Revision as of 14:02, 23 October 2010
Notebook
Construction for Lysisbox
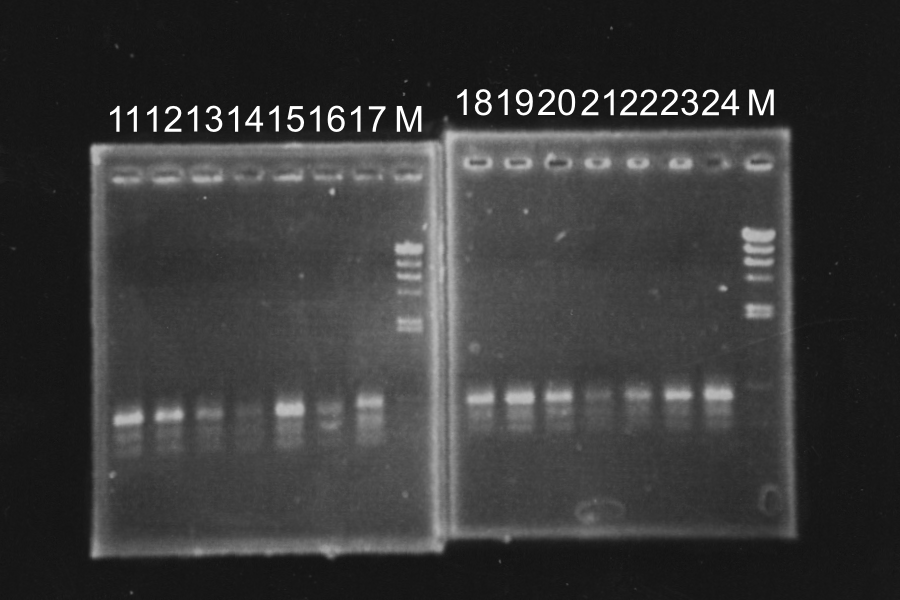
Tuesday, July 20 By: Wataru, Tomo, Yuki, Kazuya, Ken, Makoto
Transformation
| Name | Well | Sample | Competent Cells | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|---|
| J23100 | 1-18-C | 1 µL | 20 | 21 | LB (Amp+) | At 37℃, 7/20 20:50 - 7/21 17:00 | ○ |
| J23105 | 1-18-M | 1 | 20 | 21 | ○ | ||
| J23116 | 1-20-M | 1 | 20 | 21 | ○ | ||
| R0011 | 1-6-G | 1 | 20 | 21 | ○ | ||
| E0840 | 1-12-O | 1 | 20 | 21 | ○ | ||
| J06702 | 2-8-E | 1 | 20 | 21 | ○ | ||
| pSB4K5 | 1-5-G | 1 | 20 | 21 | × | ||
| B0015 | 1-23-L | 1 | 20 | 21 | LB (Kan+) | × |
A vector of pSB4K5 is Kanamycin-resistance, however, we plated it to LB plate (Amp+). And We didn't pre-culture B0015 despite its vector is Kanamycin-resistance. So, it was predicted that we will fail the transformation of pSB4K5 and B0015.
Wednesday, July 21 By: Wataru, Ken, Makoto, Takuya Y.
Culture at 37℃ from 07/21 20:50 to 07/22 17:00 and Making Master Plate
Transformation
| Name | Well | Sample | Competent Cells | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|---|
| pSB4K5 | 1-5-G | 1 µL | 20 | 21 | LB (Kan+) | At 37℃, 7/21 20:50 - 7/22 16:30 | ○ |
| B0015 | 1-23-L | 1 | 20 | 21 | ○ |
PCR for SRRz and S
| No. | Water | MgSO4 | dNTPs | 10xBuffer | Template DNA | Primer Fwd. | Primer Rev. (SRRz) | Primer Rev. (S) | KOD Plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 µL | 3 | 5 | 5 | 5 | 1.5 | 1.5 | - | 1 | 50 |
| 2 | 28 | 3 | 5 | 5 | 5 | 1.5 | 1.5 | - | 1 | 50 |
| 3 | 28 | 3 | 5 | 5 | 5 | 1.5 | - | 1.5 | 1 | 50 |
| 4 | 28 | 3 | 5 | 5 | 5 | 1.5 | - | 1.5 | 1 | 50 |
| 5 | 28 | 3 | 5 | 5 | 5 | 1.5 | 1.5 | - | 1 | 50 |
| 6 | 28 | 3 | 5 | 5 | 5 | 1.5 | 1.5 | - | 1 | 50 |
| 7 | 28 | 3 | 5 | 5 | 5 | 1.5 | - | 1.5 | 1 | 50 |
| 8 | 28 | 3 | 5 | 5 | 5 | 1.5 | - | 1.5 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30 cycles |
| 55℃ | 30s | |
| 68℃ | 4min | |
| 4℃ | forever |
Thursday, July 22 By: Wataru
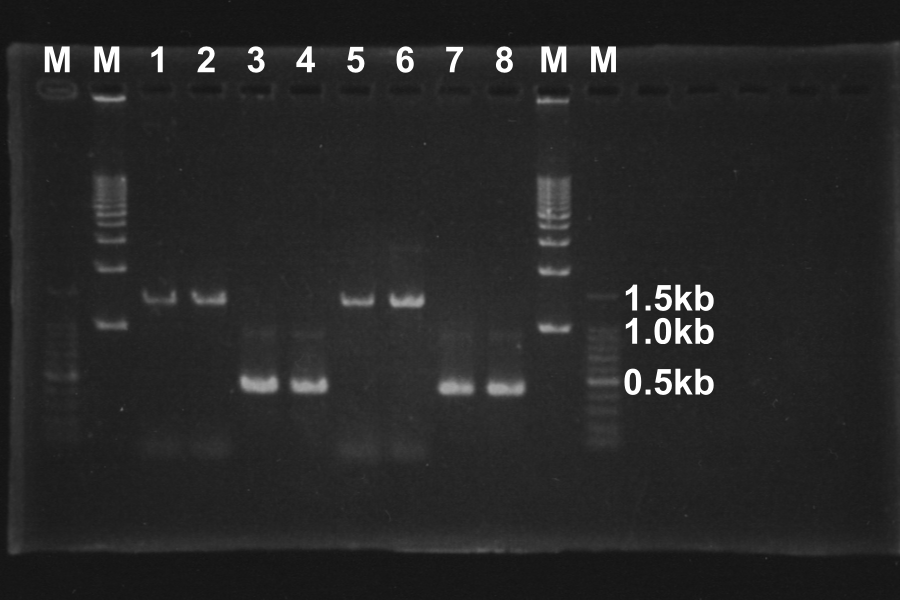
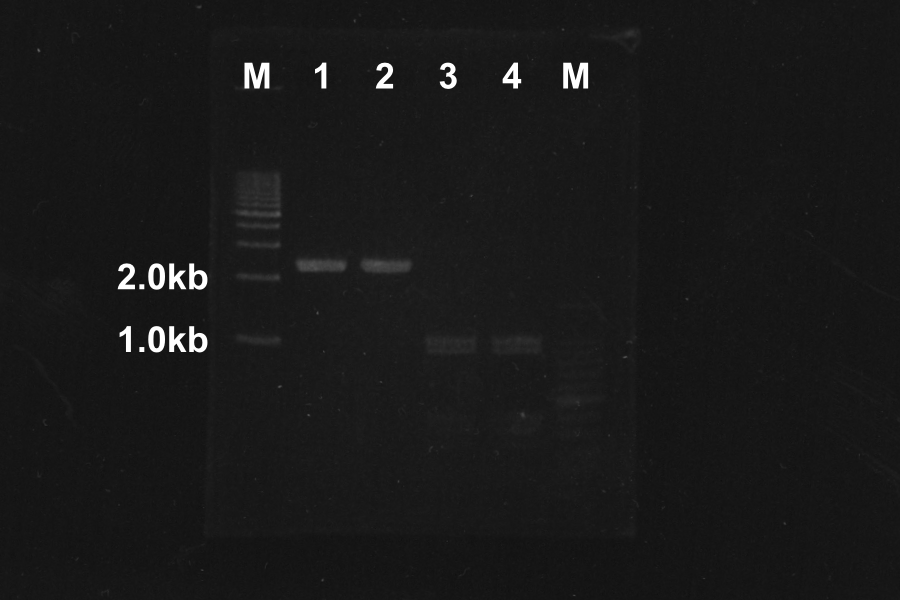
Electrophoresis (40min) of the PCR Products
| No. | Name | Length(bp) | Result |
|---|---|---|---|
| 1 | SRRz | 1386 | ○ |
| 2 | SRRz | 1386 | ○ |
| 3 | S | 442 | ○ |
| 4 | S | 442 | ○ |
| 5 | SRRz | 1386 | ○ |
| 6 | SRRz | 1386 | ○ |
| 7 | S | 442 | ○ |
| 8 | S | 442 | ○ |
Marker: 100bp, 1kb, 1kb, 100bp.
Miniprep
| Name | Concentration |
|---|---|
| J23100 | 18.5 (ng/µL) |
| J23105 | 12.5 |
| J23116 | 14.6 |
| R0011 | 8.6 |
| E0840 | 12.1 |
| J06702 | 14.7 |
The concentration of all samples was very week. Probably our shaking incubation was week.
Culture from 07/22 17:00 to 07/23 10:00 and Making Master Plates of pSB4K5 and B0015
Friday, July 23 By: Wataru, Tomo, Makoto
Miniprep
| Name | Concentration |
|---|---|
| pSB4K5 | 79.2 (ng/µL) |
| B0015 | - |
We lost B0015 by our mistake. The concentration of pSB4K5 is high, so this condition of shaking incubation is moderate.
PCR Purification
| No. | Name | Concentration | New Name |
|---|---|---|---|
| 1 | SRRz | 18.6 ng/µL | - |
| 3 | S | 77.6 | SSam7(1) |
| 5 | SRRz | 33.6 | - |
| 7 | S | 65.4 | SSam7(2) |
The concentration of sample number 1 and 5, the PCR products of S-R-Rz/Rz1, is week, so we desided to retry PCR.
PCR for SRRz
| No. | Water | MgSO4 | dNTPs | 10xBuffer | Template DNA | Primer Fwd. (SRRz) | Primer Rev. (SRRz) | KOD plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 µL | 3 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 2 | 28 | 3 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 3 | 26.5 | 4.5 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 4 | 26.5 | 4.5 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 5 | 25 | 6 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 6 | 25 | 6 | 5 | 5 | 5 | 1.5 | 1.5 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30 cycles |
| 55℃ | 30s | |
| 68℃ | 4min | |
| 4℃ | forever |
Restriction Digestion and Electrophoresis (35min) to check function of our Restriction Enzyme
| No. | Name | Sample | 10xBuffer | BSA | Enzyme | MilliQ | Total | Incubation | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | J06702 | 5 µL | 1 | 0.1 | EcoRI | 0.1 | 3.6 | 10 | At 37℃ 7/23 18:00 - 7/23 18:30 |
| 2 | J06702 | 5 | 1 | 0.1 | XbaI | 0.1 | 3.6 | 10 | |
| 3 | J06702 | 5 | 1 | 0.1 | SpeI | 0.1 | 3.6 | 10 | |
| 4 | J06702 | 5 | 1 | 0.1 | PstI | 0.1 | 3.6 | 10 | |
| 5 | J06702 | 5 | 1 | 0.1 | - | 3.7 | 10 | ||
Marker: 1kb. Comparison to No. 5 (control, circular DNA), the bands of No. 1, 2, 3, and 4 was shifted. The DNA of them was linearized by Restriction enzymes. So, our restriction enzymes work correctly.
Restriction Digestion and Ligation to insert S gene to E0840
| Name | Sample | 10xBuffer | Enzyme 1 | Enzyme 2 | MilliQ | Total | Incubation | ||
|---|---|---|---|---|---|---|---|---|---|
| SSam7(1) | 11 µL | 5 | EcoRI | 0.2 | SpeI | 0.2 | 33.6 | 50 | At 37℃ for 2h |
| SSam7(2) | 11 | 5 | EcoRI | 0.2 | SpeI | 0.2 | 33.6 | 50 | |
| E0840 | 45 | 5 | EcoRI | 0.2 | XbaI | 0.2 | 0 | 50 | |
After PCR Purification, evaporated them and diluted 3µL.
| Name | Vector | Insert | Ligation High | Total | ||
|---|---|---|---|---|---|---|
| SSam7(1)-E0840 | E0840 | 0.5µL | SSam7(1) | 0.5 | 1 | 2 |
| SSam7(2)-E0840 | E0840 | 0.5 | SSam7(2) | 0.5 | 1 | 2 |
Monday, July 26 By: Wataru, Tomonori, Makoto
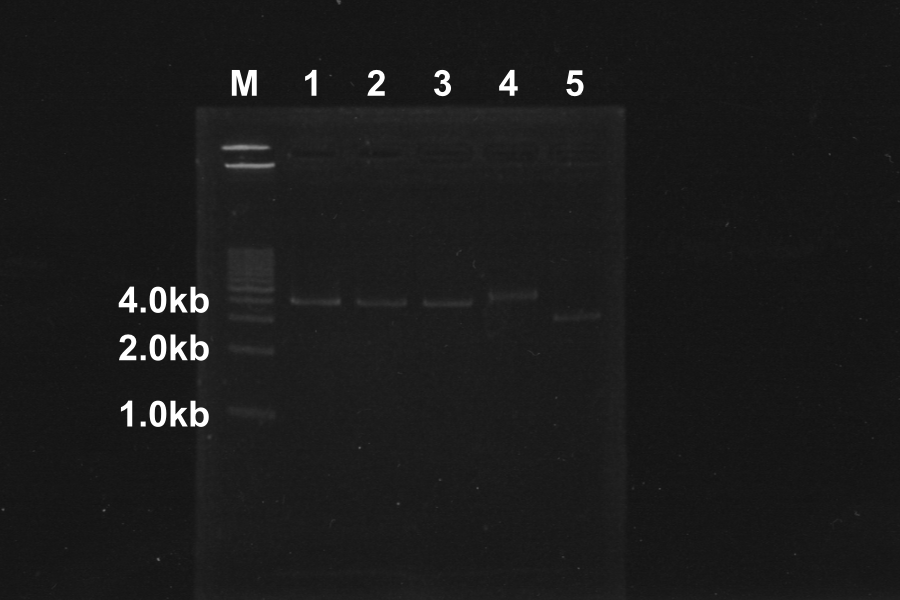
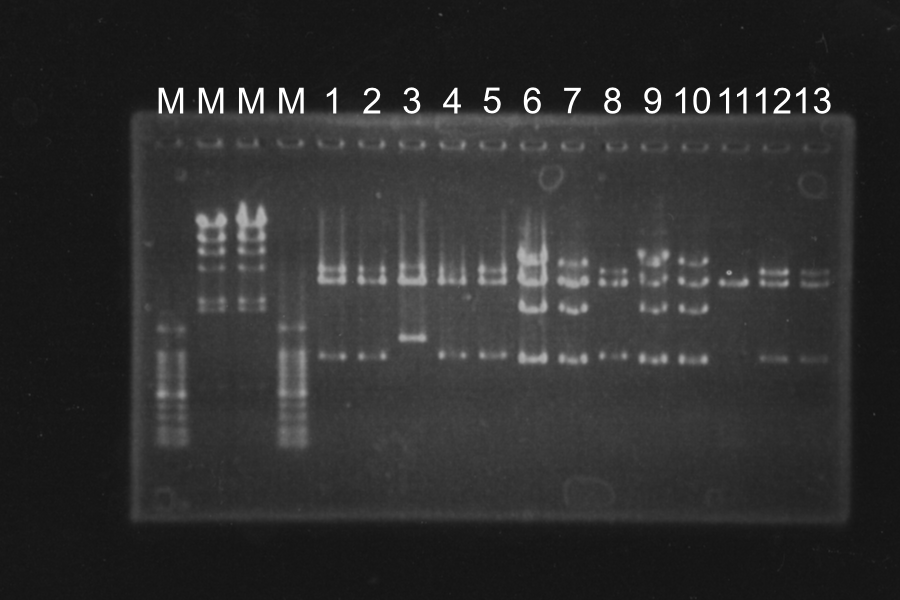
Electrophoresis of PCR Products
| No. | Name | Length(bp) | Result |
|---|---|---|---|
| 1 | SRRz | 1386 | |
| 2 | SRRz | 1386 | |
| 3 | SRRz | 1386 | |
| 4 | SRRz | 1386 | |
| 5 | SRRz | 1386 | |
| 6 | SRRz | 1386 |
Marker: 1kb. At the condition 4 (4.5µL MgSO4) and 6 (6µL MgSO4), SRRz is amplified very much. So we decided to use them.
PCR Purification
| No. | Name | Concentration | New Name |
|---|---|---|---|
| 4 | SRRZ | 51.6 ng/µL | SRRzSam7(1) |
| 5 | SRRZ | 59.3 | |
| 6 | SRRZ | 59.6 | SRRzSam7(2) |
Transformation
| Name | Well | Sample | Competent Cell | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|---|
| E0240 | 1-12-M | 1 µL | 20 | 21 | LB (Amp+) | At 37℃ 7/26 - 7/27 | × |
| I20260 | 2-17-F | 1 | 20 | 21 | LB (Kan+) | × | |
| J04450 | 1-5-E | 1 | 20 | 21 | × |
Culture of pSB4K5, E0840, and B0015
Tuesday, July 27 By: Wataru, Tomo, Kazuya, Ken, Naoi
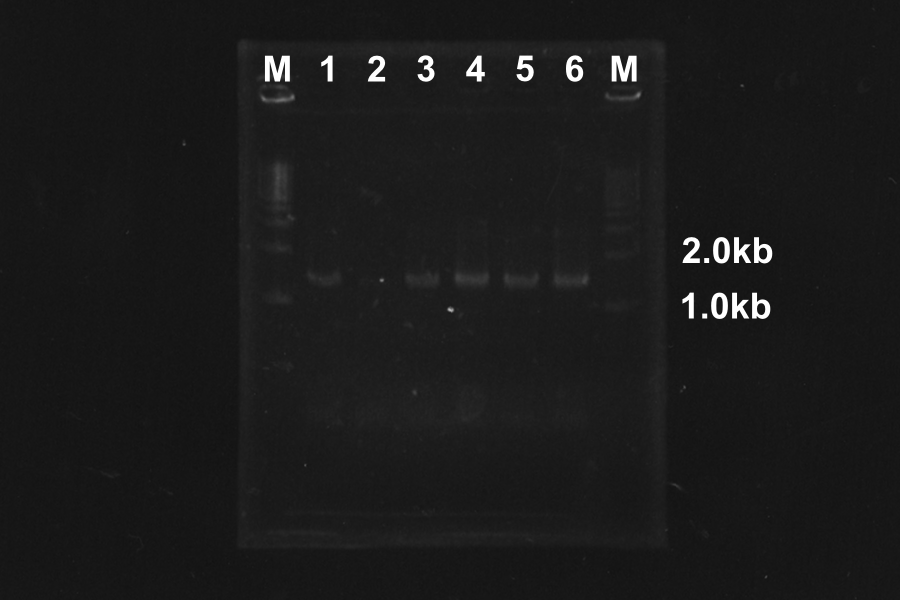
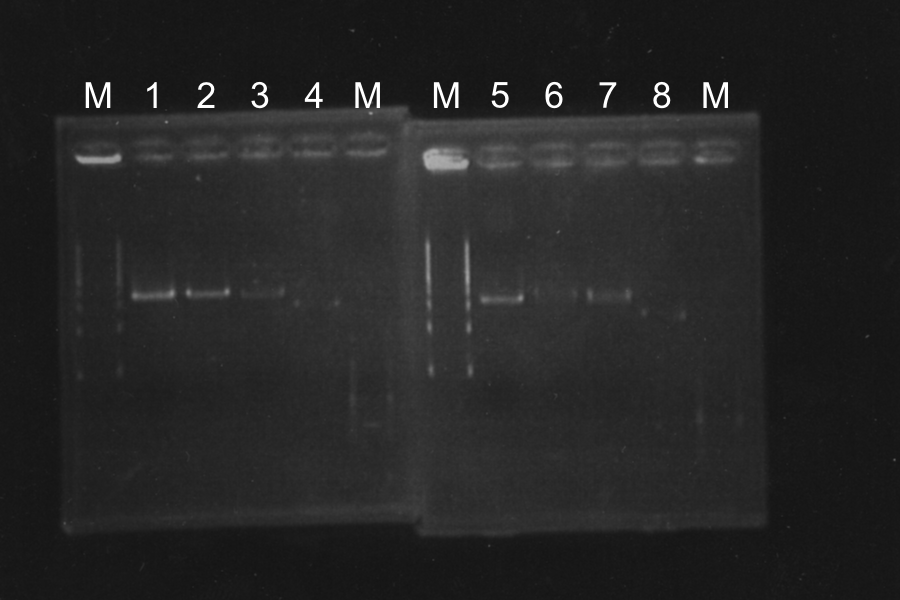
Colony PCR of SSam7-E0840 (Electrophoresis for 35min)
| No. | Name | Length | Result |
|---|---|---|---|
| 1 | SSam7(1)-E0840 | 1522 | ○ |
| 2 | SSam7(1)-E0840 | 1522 | × |
| 3 | SSam7(1)-E0840 | 1522 | ○ |
| 4 | SSam7(1)-E0840 | 1522 | × |
| 5 | SSam7(1)-E0840 | 1522 | ○ |
| 6 | SSam7(1)-E0840 | 1522 | ◎ (Use as SSam7(1)-E0840) |
| 7 | SSam7(2)-E0840 | 1522 | × |
| 8 | SSam7(2)-E0840 | 1522 | × |
| 9 | SSam7(2)-E0840 | 1522 | × |
| 10 | SSam7(2)-E0840 | 1522 | × |
| 11 | SSam7(2)-E0840 | 1522 | ◎ (Use as SSam7(2)-E0840) |
| 12 | SSam7(2)-E0840 | 1522 | ○ |
| 13 | SSam7(2)-E0840 | 1522 | ○ |
| + | E0840 | 1116 | |
| - | None |
Marker: 1kb, 100bp
Miniprep
| Name | Concentration |
|---|---|
| R0011 | 26.9 ng/µL |
| B0015 | 120.0 |
| E0840 | 120.1 |
Restriction Digestion
| Name | Sample | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | Incubation | ||
|---|---|---|---|---|---|---|---|---|---|---|
| B0015 | 30 µL | 5 | 0.5 | EcoRI | 0.4 | XbaI | 0.3 | 13.7 | 50 | At 37℃ 16:45 - 18:00 |
| SRRzSam7(1) | 40 | 5 | 0.5 | EcoRI | 0.4 | SpeI | 0.4 | 3.8 | 50 | |
| SRRzSam7(2) | 40 | 5 | 0.5 | EcoRI | 0.4 | SpeI | 0.4 | 3.8 | 50 | |
Ligation
Transformation
| Name | Sample | Competent Cells | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|
| SRRzSam7(1)-B0015 | ○ | |||||
| SRRzSam7(2)-B0015 | ○ |
Wednesday, July 28 By:
Miniprep
| Name | Concentration |
|---|---|
| SSam7(1)-E0840 | 95.5 ng/µL |
| SSam7(2)-E0840 | 98.6 |
Diluted SSam7(1)-E0840 and SSam7(2)-E0840 20 times with water, and used as template DNA.
Deletion PCR to delete a functional domain of S gene
| Water | MgSO4 | dNTPs | 10xBuffer | Primer Fwd. | Primer Rev. | Template (1) | Template (2) | KOD Plus ver.2 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| SSam7,ΔTMD1(1)-E0840 (1) | 28 µL | 3 | 5 | 5 | 1.5 | 1.5 | 5 | - | 1 | 50 |
| SSam7,ΔTMD1(1)-E0840 (2) | 28 | 3 | 5 | 5 | 1.5 | 1.5 | 5 | - | 1 | 50 |
| SSam7,ΔTMD1(2)-E0840 (1) | 28 | 3 | 5 | 5 | 1.5 | 1.5 | - | 5 | 1 | 50 |
| SSam7,ΔTMD1(2)-E0840 (2) | 28 | 3 | 5 | 5 | 1.5 | 1.5 | - | 5 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 35 cycles |
| 55℃ | 30s | |
| 68℃ | 4min | |
| 4℃ | forever |
Restriction Digestion to check the function of DpnI
| Name | Sample | fast digestion buffer | DpnI | MilliQ | Total |
|---|---|---|---|---|---|
| SSam7(1)-E0840 | 3 µL | 1 | 0.1 | 5.8 | 10 |
| SSam7(2)-E0840 | 3 | 1 | 0.1 | 5.8 | 10 |
Electrophoresis for 35min
| No. | Name | Length | Result |
|---|---|---|---|
| 1 | Not digested SSam7(1)-E0840 | 3363bp | |
| 2 | Not digested SSam7(2)-E0840 | 3363 | |
| 3 | Digested SSam7(1)-E0840 | 1021, 933, 402, 341, 258, 105, ... | |
| 4 | Digested SSam7(2)-E0840 | 1021, 933, 402, 341, 258, 105, ... |
Marker: 1kb, 100bp DpnI works correctly.
Thursday, July 29 By:
Restriction Digestion
| Name | Sample volume | Fastdigestion Buffer | Enzyme 1 | MilliQ | Total | Incubation | |
|---|---|---|---|---|---|---|---|
| SSam7,ΔTMD1(1)-E0840 (1) | 50 µL | 6 | DpnI | 0.2 | 3.8 | 60 | 07/29 09:40 - 07/29 11:00 |
| SSam7,ΔTMD1(2)-E0840 (1) | 50 | 6 | DpnI | 0.2 | 3.8 | 60 | |
Ligation and Phosphorylation
| Name | Sample | MilliQ | Ligation High | T4 Kinase | Total | Incubation |
|---|---|---|---|---|---|---|
| SSam7,ΔTMD1(1)-E0840 (1) | 2 µL | 7 | 5 | 1 | 15 | 07/29 11:30 ~ 07/29 13:00 |
| SSam7,ΔTMD1(2)-E0840 (1) | 2 | 7 | 5 | 1 | 15 |
Transformation
| Name | Sample Volume | Competent Cell | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|
| SSam7,ΔTMD1(1)-E0840 (1) | 3 µL | 30 | 33 | LB (Amp+) | 07/29 ~ 07/30 | ○ |
| SSam7,ΔTMD1(2)-E0840 (1) | 3 | 30 | 33 | ○ |
Monday, August 2 By: Wataru, Ken
Miniprep
| Name | Concentration |
|---|---|
| SSam7,ΔTMD1-E0840 (1) | 52.7 ng/µL |
| SSam7,ΔTMD1-E0840 (2) | 54.4 |
| SSam7,ΔTMD1-E0840 (3) | 89.5 |
| pSB4K5 | 50.7 |
| R0011 | 18.6 |
PCR of E0240
E0240 is very important parts to measure RPU of promoters in iGEM. However, we failed to transfect it to E.coli from parts kit of iGEM. So we decided to amplify this parts by PCR.
| Name | Water | MgSO4 | dNTPs | 10xBuffer | Primer VF2 | Primer VR | Template E0240 | KOD Pllus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| E0240(1) | 28 µL | 3 | 5 | 5 | 1.5 | 1.5 | 5 | 1 | 50 |
| E0240(2) | 28 | 3 | 5 | 5 | 1.5 | 1.5 | 5 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 35 cycles |
| 55℃ | 30s | |
| 68℃ | 4min | |
| 4℃ | forever |
Electrophoresis
PCR Purification
| Name | Concentration |
|---|---|
| E0240(1) | 42.6 ng/µL |
| E0240(2) | 55.3 |
Restriction Digestion for inserting E0240 to pSB4K5 by 3A assembly
| Name | Sample volume | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | ||
|---|---|---|---|---|---|---|---|---|---|
| E0240(1) [XP] | 30 µL | 5 | 0.5 | XbaI | 0.2 | PstI | 0.2 | 14.1 | 50 |
| E0240(2) [XP] | 30 | 5 | 0.5 | XbaI | 0.2 | PstI | 0.2 | 14.1 | 50 |
PCR Purification
| Name | Concentration | Volume |
|---|---|---|
| E0240(1) [XP] | 21.8 ng/µL | 40 µL |
| E0240(2) [XP] | 32.4 | 45 |
Stored at -20℃.
Error PCR
| Name | Water | MgSO4 | dNTPs | 10xBuffer | Primer VF2 | Primer VR | Template (1) | Template (2) | Template (3) | KOD Pllus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SSam7,ΔTMD1-E0840 (1) | 32 µL | 3 | 5 | 5 | 1.5 | 1.5 | 1 | - | - | 1 | 50 |
| SSam7,ΔTMD1-E0840 (2) | 32 | 3 | 5 | 5 | 1.5 | 1.5 | - | 1 | - | 1 | 50 |
| SSam7,ΔTMD1-E0840 (3) | 32 | 3 | 5 | 5 | 1.5 | 1.5 | - | - | 1 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 20 cycles |
| 68℃ | 4min | |
| 4℃ | forever |
Restriction Digestion of SSam7,ΔTMD1-E0840 by DpnI
Transformation
| Name | Sample | Competent Cells | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|
| SSam7,ΔTMD1-E0840 (1) | 2 µL | 20 | 22 | - | - | ○ |
| SSam7,ΔTMD1-E0840 (2) | 2 | 20 | 22 | } | ||
| SSam7,ΔTMD1-E0840 (3) | 2 | 20 | 22 | ○ |
Tuesday, August 3 By:
Culture
Picked two colonies from SSam7,ΔTMD1-E0840 (1), and SSam7,ΔTMD1-E0840 (3), and cultured at 37℃ from 08/03 to 08/04.
Miniprep
| Name | Concentration |
|---|---|
| pSB4K5 | 60.7 ng/µL |
| R0011 | 26.8 |
Restriction Digestion
| Name | Sample | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | ||
|---|---|---|---|---|---|---|---|---|---|
| R0011 [ES] | 50 µL | 6 | 0.6 | EcoRI | 0.2 | SpeI | 0.2 | 3 | 60 |
| pSB4K5 [EP] | 50 | 6 | 0.6 | EcoRI | 0.2 | PstI | 0.2 | 3 | 60 |
| E0240(1) [XP] | 50 | 6 | 0.6 | XbaI | 0.2 | PstI | 0.2 | 3 | 60 |
| E0240(2) [XP] | 50 | 6 | 0.6 | XbaI | 0.2 | PstI | 0.2 | 3 | 60 |
PCR Purification
| Name | Concentration |
|---|---|
| pSB4K5 [EP] | 39.5 ng/µL |
| E0240(1) [XP] | 21.8 |
| E0240(2) [XP] | 32.4 |
pSB4K5 [EP] is concentrated 10µL and E0240(1) [XP], E0240(2) [XP] are concentrated 1µL.
Ethanol Precipitation
After ethanol precipitation, we diluted pSB4K5 by 2µL MilliQ
Ligation
| Name | Vector | Insert 1 | Insert 2 | Ligation High | Total | Incubation | |||
|---|---|---|---|---|---|---|---|---|---|
| R0011-E0240(1) [pSB4K5] | pSB4K5 [EP] | 1 | R0011 [ES] | 1 | E0240(1) [XP] | 1 | 3 | 15 | 17:30 - 20:20 |
| R0011-E0240(2) [pSB4K5] | pSB4K5 [EP] | 1 | R0011 [ES] | 1 | E0240(2) [XP] | 1 | 3 | 15 | |
PCR of I20260
| Name | Water | MgSO4 | dNTPs | 10xBuffer | Primer VF2 | Primer VR | Template I20260 | KOD plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| I20260 (1) | 32µL | 3 | 5 | 5 | 1.5 | 1.5 | 1 | 1 | 50 |
| I20260 (2) | 32 | 3 | 5 | 5 | 1.5 | 1.5 | - | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30 cycles |
| 55℃ | 30s | |
| 68℃ | 4min | |
| 4℃ | forever |
PCR Purification
| Name | Concentration |
|---|---|
| I20260 | 40.6 ng/µL |
Restriction Digestion
| Name | Sample volume | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | ||
|---|---|---|---|---|---|---|---|---|---|
| I20260 [EP] | 45 µL | 6 | 0.6 | EcoRI | 0.2 | PstI | 0.2 | 8 | 60 |
PCR Purification
| Name | Concentration | Volume |
|---|---|---|
| I20260 [EP] | 74.1 ng/µL | 30 |
I20260 [EP] is concentrated at 7µL
Ligation
| Vector | Insert | Ligation High | Total | Incubation | |||
|---|---|---|---|---|---|---|---|
| I20260 [pSB4K5] | pSB4K5 [EP] | 1 | I20260 [EP] | 1 | 2 | 4 | 20:00-20:30 |
Transformation
| Name | Sample | Competent Cell | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|
| R0011-E0240(1) [pSB4K5] | 1 µL | 20 | 21 | LB (Kan+) | 08/03-08/04 | ○ |
| R0011-E0240(2) [pSB4K5] | 1 | 20 | 21 | ○ | ||
| I20260 [pSB4K5] | 1 | 20 | 21 | ○ |
Thursday, August 5 By:
Culture and Master Plates
pSB4K5 is inserted RFP generator. We didn't distinguish this inserted parts from low copy plasmid backbone, so self-ligated colony is red. So, white colony is correctly inserted parts.
However, white colonies and green colonies are observed in R0011-E0240(1) [pSB4K5] and R0011-E0240(2) [pSB4K5] plate. We cultured both white and green colonies.
In I20260 [pSB4K5], Many of colonies are red, but green colonies are observed. We cultured green colonies.
| Name | Color | Incubation |
|---|---|---|
| R0011-E0240(1) [pSB4K5] (1) | Green Colony | 8/5-8/6 |
| R0011-E0240(1) [pSB4K5] (2) | Green Colony | |
| R0011-E0240(1) [pSB4K5] (3) | White Colony | |
| R0011-E0240(1) [pSB4K5] (4) | White Colony | |
| R0011-E0240(2) [pSB4K5] (1) | Green Colony | |
| R0011-E0240(2) [pSB4K5] (2) | White Colony | |
| R0011-E0240(2) [pSB4K5] (3) | White Colony | |
| R0011-E0240(2) [pSB4K5] (4) | White Colony | |
| I20260 [pSB4K5] (1) | Green Colony | |
| I20260 [pSB4K5] (2) | Green Colony | |
| I20260 [pSB4K5] (3) | Green Colony |
Sequence
| Name | Concentration |
|---|---|
| SΔTMD1-E0840(1) A | 28.9 ng/µL |
| SΔTMD1-E0840(1) B | 25.3 |
| SΔTMD1-E0840(3) A | 26.6 |
| SΔTMD1-E0840(3) B | 24.0 |
As a result, deletion is succeeded, however, point mutation is failed. It is because DpnI is too little to digest all of template DNA.
Friday, August 6
Miniprep
| Name |
|---|
| R0011-E0240(1) [pSB4K5] (1) |
| R0011-E0240(1) [pSB4K5] (2) |
| R0011-E0240(1) [pSB4K5] (3) |
| R0011-E0240(1) [pSB4K5] (4) |
| R0011-E0240(2) [pSB4K5] (1) |
| R0011-E0240(2) [pSB4K5] (2) |
| R0011-E0240(2) [pSB4K5] (3) |
| R0011-E0240(2) [pSB4K5] (4) |
| I20260 [pSB4K5] (1) |
| I20260 [pSB4K5] (2) |
| I20260 [pSB4K5] (3) |
Restriction Digestion
| Name | Sample | 2 buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | ||
|---|---|---|---|---|---|---|---|---|---|
| R0011-E0240(1) [pSB4K5] (1) [EP] | 50 µL | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| R0011-E0240(1) [pSB4K5] (2) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| R0011-E0240(1) [pSB4K5] (3) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| R0011-E0240(1) [pSB4K5] (4) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| R0011-E0240(2) [pSB4K5] (1) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| R0011-E0240(2) [pSB4K5] (2) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| R0011-E0240(2) [pSB4K5] (3) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| R0011-E0240(2) [pSB4K5] (4) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| I20260 [pSB4K5] (1) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| I20260 [pSB4K5] (2) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
| I20260 [pSB4K5] (3) [EP] | 50 | 6 | 0.6 | EcoRI | 0.3 | PstI | 0.3 | 2.8 | 60 |
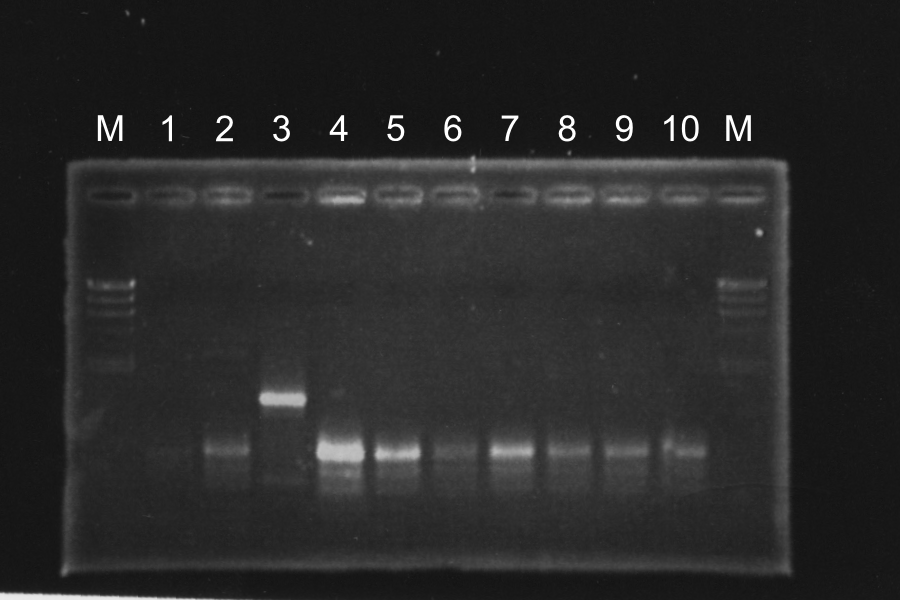
==Electrophoresis
| No. | Name | Length | Results |
|---|---|---|---|
| 1 | I20260 [pSB4K5] (1) [EP] | 960, 4339 | |
| 2 | I20260 [pSB4K5] (2) [EP] | 960, 4339 | |
| 3 | I20260 [pSB4K5] (3) [EP] | 960, 4339 | |
| 4 | R0011-E0240(1) [pSB4K5] (1) [EP] | 980 3378 | ○ |
| 5 | R0011-E0240(1) [pSB4K5] (2) [EP] | 980 3378 | ○ |
| 6 | R0011-E0240(1) [pSB4K5] (3) [EP] | 980 3378 | } |
| 7 | R0011-E0240(1) [pSB4K5] (4) [EP] | 980 3378 | } |
| 8 | R0011-E0240(2) [pSB4K5] (1) [EP] | 980 3378 | ○ |
| 9 | R0011-E0240(2) [pSB4K5] (2) [EP] | 980 3378 | } |
| 10 | R0011-E0240(2) [pSB4K5] (3) [EP] | 980 3378 | } |
| 11 | R0011-E0240(2) [pSB4K5] (4) [EP] | 980 3378 | } |
| 12 | I20260 [pSB4K5] (1) [EP] | 960, 4339 | ○ |
| 13 | I20260 [pSB4K5] (2) [EP] | 960, 4339 | ○ |
White colonies are not inserted R0011 but its vector. Top10 we used are deleted Lac operon. Then, correctly inserted parts is green because of the lack of lacI gene.
Error PCR (Retry)
| Name | Water | MgSO4 | dNTPs | 10xBuffer | Primer VF2 | Primer VR | Template SSam7,ΔTMD1-E0840 failed (50ng/µL) | KOD plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| SSam7,ΔTMD1-E0840 (1) | 32 | 3 | 5 | 5 | 1.5 | 1.5 | 1 | 1 | 50 |
| SSam7,ΔTMD1-E0840 (2) | 32 | 3 | 5 | 5 | 1.5 | 1.5 | 1 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 25 cycles |
| 68℃ | 4min | |
| Add DpnI 2µL | ||
| Incubate | 1h | |
| 4℃ | forever | |
Transformation
| Name | Well | Sample | Competent Cell | Total | Plate | Incubation | Colony |
|---|---|---|---|---|---|---|---|
| SSam7,ΔTMD1-E0840 (1) | - | 4 µL | 50 | 54 | LB (Kan+) | 08/06-08/09 | ○ |
| SSam7,ΔTMD1-E0840 (2) | - | 4 | 50 | 54 | ○ | ||
| I20260 | 2-17-F | 2 | 50 | 52 | ○ | ||
| 2-I-5 | 2 | 50 | 52 | LB (Amp+) | ○ |
Monday, August 9 By: Wataru, Tomonori, Ken, Takuya
Miniprep
| Name | concentration |
|---|---|
| I20260 [pSB4K5] | 116.2 ng/µL |
| R0011-E0240 [pSB4K5] | 146.6 |
Transfotrmation
| Sample | Sample | Competent Cell | Total | Plate | Incuvation | Results | |
|---|---|---|---|---|---|---|---|
| I20260 [pSB4K5] | 2 µL | KRX | 50 | 52 | LB (Kan+) | 08/09 18:00-08/10 12:00 | ○ |
| R0011-E0240 [pSB4K5] | 2 | KRX | 50 | 52 | ○ | ||
Restriction Eigestion and Ethanol Precipitation
To use R0011 for next ligation, we digested it by EcoRI and PstI
| Name | Sample | 10x Buffer | BSA | Enzyme 1 | Enzyme 2 | MilliQ | Total | Incubation | ||
|---|---|---|---|---|---|---|---|---|---|---|
| R0011 [EP] | 50 | 6 | 0.6 | EcoRI | 0.5 | PstI | 0.5 | 2.4 | 60 | At 37℃ 08/09 16:20-18:20 |
After restriction enzyme digestion, we did ethanol precipitation.
Ligation and Transformation
| Sample | Competent cell | Total | Plate | Incuvation | Colony | ||
|---|---|---|---|---|---|---|---|
| R0011 [pSB4K5, KRX] | 2 µL | KRX | 50 | 52 | LB (Kan+) | 08/09 20:00-08/10 09:00 | ○ |
| R0011 [pSB4K5</partrinfo>, C2] | 2 | C2 | 50 | 52 | ○ | ||
====Tuesday, August 10 By: Wataru, Tomonori, Ken, Fumitaka
Culture
Cultured I20260 [pSB4K5, R0011-E0240 [pSB4K5], R0011 [pSB4K5, KRX], and R0011 [pSB4K5</partrinfo>, C2].
Minprep
| Name | Concentration |
|---|---|
| SSam7,ΔTMD1-E0840 (1-1) | 9.9 ng/µL |
| SSam7,ΔTMD1-E0840 (1-2) | 27.3 |
| SSam7,ΔTMD1-E0840 (2-1) | 43.2 |
| SSam7,ΔTMD1-E0840 (2-2) | 34.7 |
Culture and Master Plate
At 37℃ 08/09 18:00-08/10 9:00
Wednesday, August 11 By: Wataru, Naoi, Ken, Takuya
| No. | Medium | Cloud | Incubation |
|---|---|---|---|
| 1 | Kanamycin | ○ | At 37℃, 08/10 20:00-08/11 9:00 |
| Ampicillin | } | ||
| 2 | Kanamycin | ○ | |
| Ampicillin | ○ | ||
| 3 | Kanamycin | ○ | |
| Ampicillin | } | ||
| 4 | Kanamycin | ○ | |
| Ampicillin | } | ||
| 5 | Kanamycin | ○ | |
| Ampicillin | } | ||
| 6 | Kanamycin | ○ | |
| Ampicillin | ○ | ||
| 7 | Kanamycin | ○ | |
| Ampicillin | } |
Discussion: About sample 1, 3, 4, 5 and 7, lac promoter was correctly inserted in low copy plasmid. About sample 2 and 6, low copy plasmid and vector derived from lac promoter were ligated. We decided to use sample 1 or 3.
Miniprep of R0011 [pSB4K5, C2], SRRz 1', 3'
| Name | Concentration |
|---|---|
| R0011 [pSB4K5, C2] (1) | 31.2 ng/µL |
| R0011 [pSB4K5, C2] (3) | 29.9 |
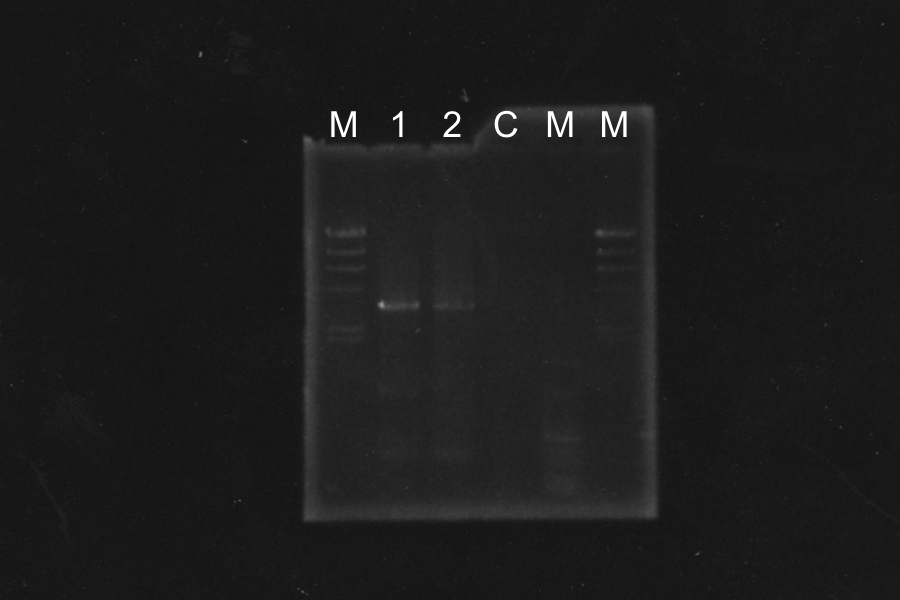
Restriction Digestion and electrophoresis of R0011 [pSB4K5, C2]
| Name | EcoRI | PstI |
|---|---|---|
| 1 | 0.2 | - |
| 2 | - | 0.2 |
| 3 | 0.2 | 0.2 |
| N | - | - |
| No. | Name | Length | Results |
|---|---|---|---|
| 1 | R0011 [pSB4K5, C2] (1-1) | ||
| 2 | R0011 [pSB4K5, C2] (1-2) | ||
| 3 | R0011 [pSB4K5, C2] (1-3) | ||
| 4 | R0011 [pSB4K5, C2] (1-N) | ||
| 5 | R0011 [pSB4K5, C2] (2-1) | ||
| 6 | R0011 [pSB4K5, C2] (2-2) | ||
| 7 | R0011 [pSB4K5, C2] (2-3) | ||
| 8 | R0011 [pSB4K5, C2] (2-N) |
Each enzyme correctly cut samples.
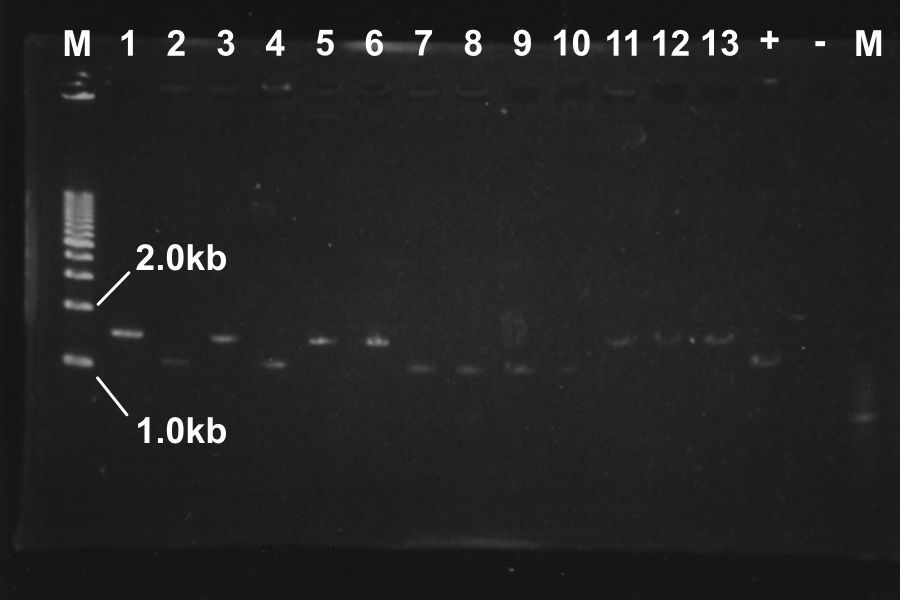
Screening PCR of SRRz
| No. | Name | Results |
|---|---|---|
| 1 | None | |
| 2 | Control B0015 | |
| 3 | Control J06702 | |
| 4 | Control B0015 | |
| 5-24 | SRRz-B0015 | } |
Marker: Lambda Marker
Discussion: All of the sample were self-ligation of DT. SRRz weren't inserted.
Thursday, August 12 By: Wataru, Ken
Restriction Digestion and electrophoresis of B0015
| Name | Template | 10xbuffer | 100xbuffer | EcoRI | XbaI 1 | XbaI 2 | SpeI | PstI 1 | PstI 2 | Water | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 1 | 0.1 | 0.2 | - | - | - | - | - | 5.7 | 10 |
| 2 | 3 | 1 | 0.1 | - | 0.2 | - | - | - | - | 5.7 | 10 |
| 3 | 3 | 1 | 0.1 | - | - | 0.2 | - | - | - | 5.7 | 10 |
| 4 | 3 | 1 | 0.1 | - | - | - | 0.2 | - | - | 5.7 | 10 |
| 5 | 3 | 1 | 0.1 | - | - | - | - | 0.2 | - | 5.7 | 10 |
| 6 | 3 | 1 | 0.1 | - | - | - | - | - | 0.2 | 5.7 | 10 |
| N | 3 | 1 | 0.1 | - | - | - | - | - | - | 5.9 | 10 |
Maker: Lambda, 100bp
Discussion: Each enzyme correctly cut each sample and was active.
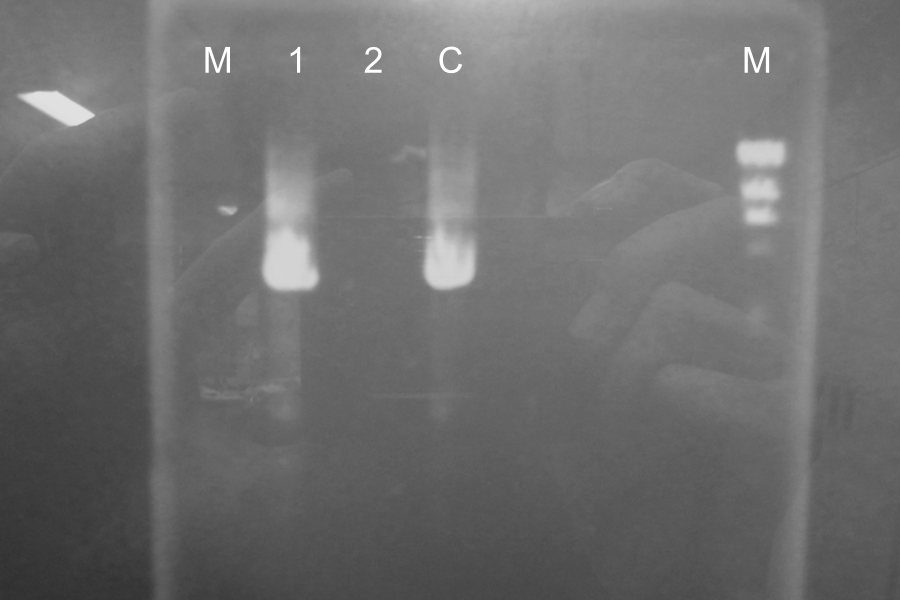
====Thursday, August 19 By: Wataru, Tomo, Ken
Miniprep of SSam7,ΔTMD1-E0840
| Name | Concentration |
|---|---|
| SSam7,ΔTMD1-E0840 | 29.6 ng/µL |
Point mutation PCR of SSam7,ΔTMD1-E0840
| Name | Template | 10xbuffer | dNTPs | MgSO4 | Primer Fwd. | Primer Rev. | MilliQ | KOD plus ver.2 | Total |
|---|---|---|---|---|---|---|---|---|---|
| SΔTMD1-E0840 (1) | 1.5 | 5 | 5 | 3 | 1.5 | 1.5 | 31.5 | 1 | 50 |
| SΔTMD1-E0840 (2) | 1.5 | 5 | 5 | 3 | 1.5 | 1.5 | 31.5 | 1 | 50 |
| Control | 1.5 | 5 | 5 | 3 | 1.5 | 1.5 | 32.5 | - | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30cycles |
| 55℃ | 30s | |
| 68℃ | 3.5min | |
| 4℃ | forever |
Restriction Digestion by DpnI from 17:50 to 18:50
Electrophoresis
| Name |
|---|
| SΔTMD1-E0840 (1) |
| SΔTMD1-E0840 (2) |
| Control |
Marker: Lambda, 100bp
Ligation and Transformation
| Name | Colony |
|---|---|
| SΔTMD1-E0840 (1) | ○ |
| SΔTMD1-E0840 (2) | ○ |
| Control | } |
Friday, August 20 By: Wataru, Ken
Making Culture and Master Plate of SΔTMD1-E0840
Miniprep
| Name | Concentration |
|---|---|
| B0015 | 41.1 ng/µL |
PCR of SRRz
| Name | 10xBuffer | MgS04 | dNTP | Template | Primer Fwd. | Primer Rev. | MilliQ | KOD plus ver.2 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|
| SRRzSam7 (1) | 5 µL | 3 | 5 | 5 | F1 | 1.5 | 1.5 | 28 | 1 | 50 |
| SRRzSam7 (2) | 5 | 3 | 5 | 5 | F2 | 1.5 | 1.5 | 28 | 1 | 50 |
| SRRzSam7 (3) | 5 | 3 | 5 | 5 | F1 | 1.5 | 1.5 | 28 | 1 | 50 |
| SRRzSam7 (4) | 5 | 3 | 5 | 5 | F2 | 1.5 | 1.5 | 28 | 1 | 50 |
| SRRzSam7 (5) | 5 | 3 | 5 | 5 | F1 | 1.5 | 1.5 | 28 | 1 | 50 |
| SRRzSam7 (6) | 5 | 3 | 5 | 5 | F2 | 1.5 | 1.5 | 28 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30cycles |
| 55℃ | 30s | |
| 68℃ | 2min | |
| 4℃ | forever |
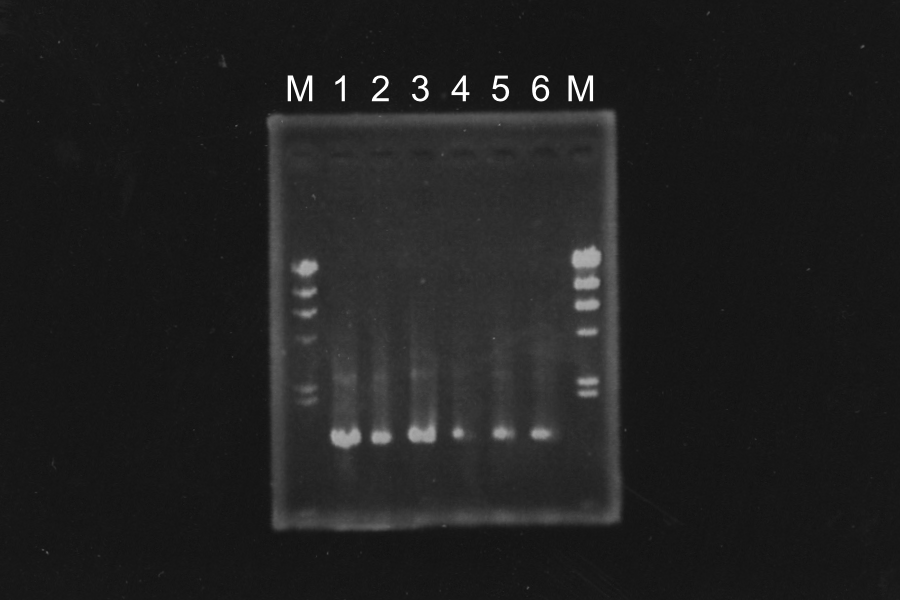
Electrophoresis
| Name |
|---|
| SRRzSam7 (1) |
| SRRzSam7 (3) |
| SRRz Sam7(5) |
| SRRzSam7 (2) |
| SRRzSam7 (4) |
| SRRzSam7 (6) |
Discussion: Primer F1 might be better than F2, because the bands of 1, 2 and 3 were clearer. We decided to use sample 1 and 3. Their bands were clearer in the three.
PCR Purification
| Name | Concentration |
|---|---|
| SRRzSam7 (1) | 134.0 ng/µL |
| SRRzSam7 (3) | 69.0 |
Restriction Digestion
| Name | Sample | 10xBuffer | 100xBuffer | EcoRI | XbaI | SpeI | MilliQ | Total | Incubation |
|---|---|---|---|---|---|---|---|---|---|
| B0015 [EX] | 50 µL | 6 | 0.6 | 0.4 | 0.4 | - | 2.6 | 60 | 17:45-18:45 |
| SRRzSam7 (1) [EP] | 50 | 6 | 0.6 | 0.4 | - | 0.4 | 2.6 | 60 | |
| SRRzSam7 (3) [EP] | 50 | 6 | 0.6 | 0.4 | - | 0.4 | 2.6 | 60 |
Purification
| Name | Concentration |
|---|---|
| SRRzSam7 (1) [EP] | 109.0 ng/µL |
| SRRzSam7 (2) [EP] | 110.0 |
| B0015 | 25.5 |
Ligation and Transformation
Monday, August 23 By: Wataru, Tomo, Ken, Fumitaka, Tasuku
Miniprep
| Sample number | Concentration |
| SΔTMD1-E0840 (1-1) | 58.9 ng/µL |
| SΔTMD1-E0840 (2-2) | 49.9 |
Sequence
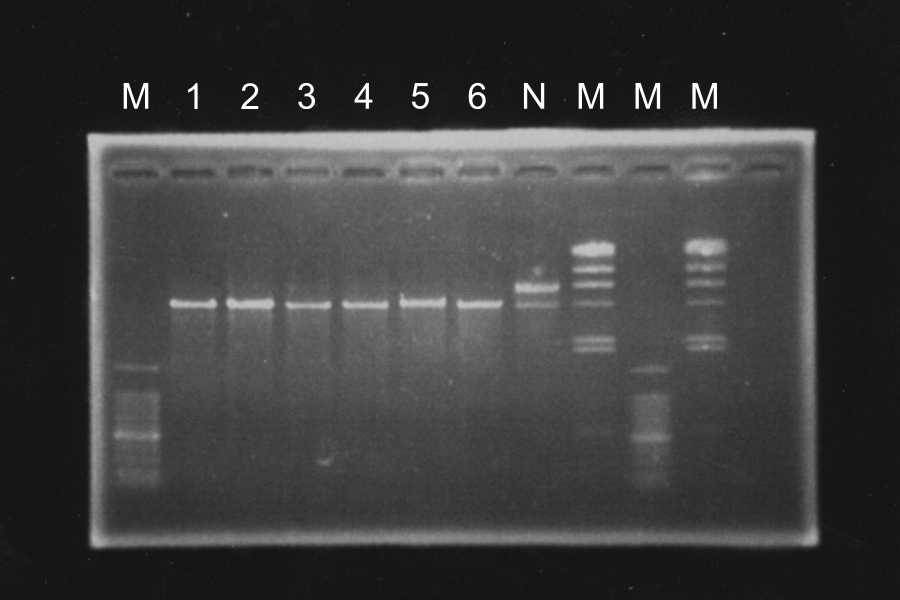
Sample: SΔTMD1-E0840 (1-1). SΔTMD1-E0840 (2-2), I20260 [pSB4K5] Discussion: The sequencing was in success and the results were desirable. It meant the point mutation was succeeded and sequence of I20260 [pSB4K5] was confirmed. We decided to use SΔTMD1-E0840.
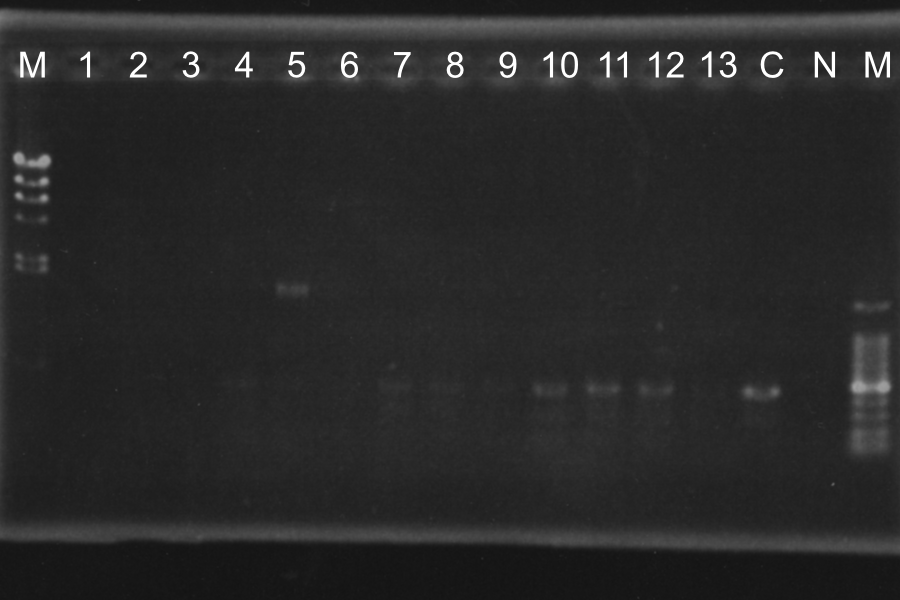
Screening PCR of SRRzSam7-B0015
| 90℃ | 10min | |
| 94℃ | 30s | 35cycles |
| 50℃ | 30s | |
| 72℃ | 1.5min | |
| 72℃ | 4min | |
| 4℃ | hold |
| No. | Name |
|---|---|
| 1-13 | SRRzSam7-B0015 |
| C | Control: B0015 |
| N | None |
Marker: Lambda, 100bp
Discussion: We found the band; about 200bp, and it meant the lligation was completed successfully.
Deletion PCR of SΔTMD1-E0840 (2-2)
| Name | Sample | 10xBuffer | dNTPs | Primer Fwd. | Primer Rev. | Template | Water | KOD-plus- | Total |
|---|---|---|---|---|---|---|---|---|---|
| rrSΔTMD1-E0840 (1) | 5 µL | 5 | 1.5 | 1.5 | 1 | 35 | 1 | 50 | |
| rrSΔTMD1-E0840 (2) | 5 | 5 | 1.5 | 1.5 | 1 | 35 | 1 | 50 | |
| rrSΔTMD1-E0840 (Control) | 5 | 5 | 1.5 | 1.5 | 1 | 35 | - | 50 |
| 94℃ | 2min | |
| 94℃ | 10s | 35cycles |
| 56℃ | 30s | |
| 68℃ | 3.5min | |
| 4℃ | forever |
Restriction Digestion (DpnI)
| Sample | DpnI | Total | Incubation |
|---|---|---|---|
| 25 µL | 1 | 26 | 19:00-20:10 |
Ligation
| Name | Sample | Water | Ligation high | T4 Kinase | Total | Incubation |
|---|---|---|---|---|---|---|
| rrSΔTMD1-E0840 (1) | 3 µL | 6 | 5 | 1 | 15 | 20:15-21:15 |
| rrSΔTMD1-E0840 (2) | 3 | 6 | 5 | 1 | 15 | 20:15-21:15 |
| rrSΔTMD1-E0840 (Control) | 3 | 6 | 5 | 1 | 15 |
Transformation
Tuesday, August 24 By: Ken, Tomo, Tasuku, Takuya
Retry of deletion PCR of SΔTMD1-E0840
| Name | Sample | 10xBuffer | dNTPs | MgSO4 | Primer1 | Primer2 | Template | Water | KOD-plus- | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| rrSΔTMD1-E0840 (1) | 5 µL | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 | |
| rrSΔTMD1-E0840 (2) | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 | |
| Control | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
| 94℃ | 2min | |
| 94℃ | 10s | 35cycles |
| 58℃ | 30s | |
| 68℃ | 3.5min | |
| 4℃ | hold |
Restriction Digestion (DpnI)
14:15-15:15
Electrophoreis
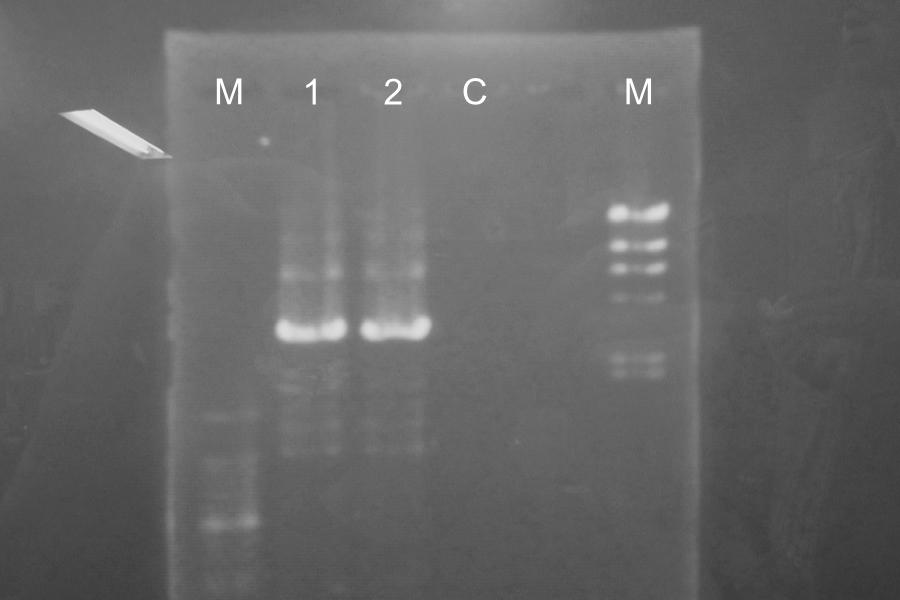
| Lane | Name |
|---|---|
| 1 | rrSΔTMD1-E0840 (1) |
| 2 | rrSΔTMD1-E0840 (3) |
| C | rrSΔTMD1-E0840 (Control) |
Marker: 100bp, Lambda.
We found the band of sample 1 and 2 about 3000bp and there wasn't the band of sample control. So, we confirmed the PCR and Restriction Digestion were completed successfully.
Ligation
Point mutation of SRRz
| Name | 10x | dNTPs | MgSO4 | Primer1 | Primer2 | Template | Water | KOD-plus- | total |
|---|---|---|---|---|---|---|---|---|---|
| SRRzSam7-B0015 (1) | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
| SRRzSam7-B0015 (2) | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
| SRRzSam7-B0015 (Control) | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 32 | 1 | 50 |
| 94℃ | 2min | |
| 98℃ | 10s | 30cycles |
| 55℃ | 30s | |
| 68℃ | 4min | |
| 4℃ | hold |
Restriction Digestion (DpnI), Electrophoresis and Ligation
We could find point mutation PCR and restriction enzyme of DpnI was done.
PCR of E0240
| Sample | 10xBuffer | dNTPs | MgSO4 | VF2 | VR | Template | Water | KOD-plus- | Total |
|---|---|---|---|---|---|---|---|---|---|
| E0240 (1) | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 31.5 | 1 | 50 |
| E0240 (2) | 5 | 5 | 3 | 1.5 | 1.5 | 1 | 31.5 | 1 | 50 |
PCR Purification
| Name | Concentration |
|---|---|
| E0240 (1) | 5.5 x 50 ng/µL |
| E0240 (2) | 5.2 x 50 |
Restriction Digestion (EcoRI, PstI) and Gel Extraction
| Name | Concentration |
|---|---|
| E0240 (1) | 28.8 ng/µL |
| E0240 (2) | 26.4 |
Transformation
Wednesday, August 25 By:Ken, Tomo, Kazuya, Tasuku, Takuya
Making culture and Master plate
| Name | Colony |
|---|---|
| rrSΔTMD1-E0840 (1) | ○ |
| rrSΔTMD1-E0840 (2) | ○ |
| rrSΔTMD1-E0840 (Control) | } |
| SRRzSam7-B0015 (1) | ○ |
| SRRzSam7-B0015 (2) | ○ |
| SRRzSam7-B0015 (Control) | } |
Miniprep
| Name | Concentration |
|---|---|
| pSB4K5 | 29.0 ng/µL |
Restriction Digestion
| Sample name | Template | 10xbuffer | 100xbuffer | EcoRI | SpeI | PstI | Water | Total |
|---|---|---|---|---|---|---|---|---|
| pSB4K5 | 50 | 6 | 0.6 | 0.4 | 0.4 | - | 2.6 | 60 |
| R0011 [pSB4K5] | 10 | 4 | 0.4 | - | 0.3 | 0.3 | 25 | 40 |
Purification
| Sample Name | Concentration |
| pSB4K5 | 18.4 ng/µL |
| R0011 [pSB4K5] | 8.6 |
Ligation of E0240 and pSB4K5, Transformation
====Thursday, August 26 By:Ken, Tomo, Kazuya, Tasuku, Takuya, Fumitaka
Miniprep
| Sample name | Concentration |
| J23116 (RPU0.7) | 44.5 ng/µL |
Restriction Digestion
| Name | Template | 10xbuffer | 100xbuffer | SpeI | PstI | Water | Total |
|---|---|---|---|---|---|---|---|
| J23116 (RPU0.7) | 25 | 4 | 0.4 | 0.3 | 0.3 | 10 | 40 |
Purification of
| Name | Concentration |
|---|---|
| J23116 (RPU0.7) | 49.8 ng/µL |
Friday, August 27 By: Ken, Tomo, Kazuya, Fumitaka
Making master plate of E0240 [pSB4K5]
| Sample Name | Concentration |
| rrSΔTMD1-E0840 (1-2) | 20.9 ng/µL |
| SRRz-B0015 (1-1) | 16.4 |
Restriction Digestion
| Name | Template | 10xbuffer | 100xbuffer | XbaI | PstI | Water | Total | Incubation |
|---|---|---|---|---|---|---|---|---|
| rrSΔTMD1-E0840 (1-2) | 45 µL | 6 | 0.6 | 0.3 | 0.3 | 7.8 | 60 | 13:20-14:20 |
| SRRz-B0015 (1-1) | 45 | 6 | 0.6 | 0.3 | 0.3 | 7.8 | 60 |
Purification
| rrSΔTMD1-E0840 (1-2) | 44.7 ng/µL |
| SRRz-B0015 (1-1) | 56.1 |
Ligation and Transformation
| Name |
|---|
| R0011-rrSΔTMD1-E0840 (1-2) |
| J23116 (RPU0.7)- rrSΔTMD1-E0840 (1-2) |
| R0011-SRRz-B0015 (1-1) [pSB4K5] |
Monday, August 30 By: Tomonori, Kazuya, Tasuku, Ken
Making culture and Master plate
| Name | Colony | R0011-rrSΔTMD1-E0840 | Many colonies |
|---|---|---|---|
| R0011-rrSΔTMD1-E0840 (Control) | Some colonies | ||
| J23116 (RPU0.7)- rrSΔTMD1-E0840 | Many colonies | ||
| J23116 (RPU0.7)- rrSΔTMD1-E0840 (Control) | Many colonies | ||
| R0011-SRRz-B0015 (1-1) [pSB4K5] | No colony | ||
| R0011-SRRz-B0015 (1-1) [pSB4K5] (Control) | No colony |
Discussion: There ware some colonies, which emitted green light, on the plate 1. So, we cultured those colonies on master plate. On the plate 5 and 6, even though we used KRX, which is able to repress lac promoter, colonies might be dead. However, we still have to do some experience so that we confirm lac promoter cannot repress enough and E. coli cannot survive.
====Tuesday, August 31 By: Tomonori, Takuya Y., Kazuya, Tasuku, Takuya, Ken
Miniprep
| Name | Concentration |
|---|---|
| J23105 (RPU0.3) | 48.5 ng/µL |
| R0011-rrSΔTMD1-E0840 | 107.3 |
Restriction Digestion
Gel Extraction of R0011-rrSΔTMD1-E0840 (Electrophoresis for 45min)
Discussion: There were two band at the bottom of the gel. It was too long -45min-, and insert and vector might be contaminated. But we went on next operation.
Purification of J23105 (RPU0.3) and R0011-rrSΔTMD1-E0840
| Name | Concentration | J23105 (RPU0.3) | 5.8 ng/µL |
|---|---|---|---|
| R0011-rrSΔTMD1-E0840 | 7.8 |
Ligation and Transformation
| Insert | Vector |
| R0011-rrSΔTMD1-E0840 | J23105 (RPU0.3) |
Wednesday, September 1 By: Tomonori, Kazuya, Tasuku, Fumitaka, Ken
Making culture and Master plate
| Name | Colony |
|---|---|
| R0011-rrSΔTMD1-E0840-J23105 (RPU0.3) | Many colonies |
| R0011-rrSΔTMD1-E0840-J23105 (RPU0.3) (Control) | Many colonies |
Screenig PCR of R0011-rrSΔTMD1-E0840-J23105 (RPU0.3)
- Sample: 1-13
- Control: Positive (B0015)
- Maker: lambda, 100
Discussion: All of the sample except sample 10 might be self-ligation products of J23105 (RPU0.3).
Miniprep
| SRRz-B0015 (1-1) | 33.8 ng/µL |
| pSB4K5 | 56.0 |
Restriction Digestion of SRRz and pSB4K5
| Name | Template | 10xbuffer | 100xbuffer | EcoRI | PstI | Water | Total | Incubation |
|---|---|---|---|---|---|---|---|---|
| SRRz-B0015 (1-1) | 20 µL | 4 | 0.4 | 0.3 | 0.3 | 15 | 40 | 13:25-14:30 |
| pSB4K5 | 20 | 4 | 0.4 | 0.3 | 0.3 | 15 | 40 |
Purification
| SRRz-B0015 (1-1) | 6.5 ng/µL |
| pSB4K5 | 16.8 |
=Ligation and transformation
- Insert: SRRz-B0015 (1-1)
- Vector: pSB4K5
Thursday, September 2 By: Tomonori, Tomo, Takuya, Ken
Making culture and Master plate
| SRRz-B0015 (1-1) [pSB4K5] | 13 colonies |
| SRRz-B0015 (1-1) [pSB4K5] (Control) | 13 colonies |
Screening PCR of rSRRz low
Sample: (1-13) SRRz-B0015 (1-1) [pSB4K5] Maker: Lambda, 100 Control: Positive (B0015), Neganive Discussion: From sample 1, two vectors might be ligated. Sample 3 and 4, SRRz-B0015 might be inserted in low copy plasmid correctly. Sample 11, it might be the self-ligation product of low copy plasmid. Anyway, we decided to culture those 4 colonies on master plate.
Friday, September 3 By: Tomonori, Tomo, Kazuya, Tasuku, Fumitaka, Ken
Making culture
- R0011-rrSΔTMD1-E0840 (1)
- R0011-rrSΔTMD1-E0840 (3)
- rrSΔTMD1-E0840 (1-1)
- rrSΔTMD1-E0840 (1-2)
- SRRz-B0015 (1-1) [pSB4K5]
- SRRz-B0015 (1-2) [pSB4K5]
- R0011-E0240 [pSB4K5]
 "
"