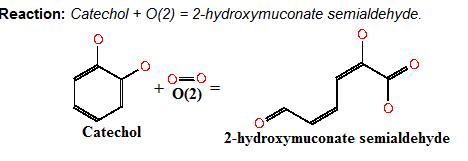Team:Imperial College London/Modules/Fast Response
From 2010.igem.org
(→C23O) |
|||
| Line 40: | Line 40: | ||
Catechol 2,3-dioxygenase (also known as C23O or MPC) catalyses the conversion of a colourless substrate (catechol or substituted catechols) into a bright yellow product (2-hydroxymuconic semialdehyde) within seconds of substrate addition. We performed our specific plate assays using a 100mM stock solution of catechol and via Pasteur pipette covering individual colonies in the solution. Liquid assays were performed with addition of a pre-made known concentration of catechol typically 0.05-0.35M diluted with DDH20. | Catechol 2,3-dioxygenase (also known as C23O or MPC) catalyses the conversion of a colourless substrate (catechol or substituted catechols) into a bright yellow product (2-hydroxymuconic semialdehyde) within seconds of substrate addition. We performed our specific plate assays using a 100mM stock solution of catechol and via Pasteur pipette covering individual colonies in the solution. Liquid assays were performed with addition of a pre-made known concentration of catechol typically 0.05-0.35M diluted with DDH20. | ||
It was convenient that a Biobrick for the XylE gene was already present in the registry and we used this along with biobrick cloning methods to generate many of our XylE final and testing constructs. | It was convenient that a Biobrick for the XylE gene was already present in the registry and we used this along with biobrick cloning methods to generate many of our XylE final and testing constructs. | ||
| - | An issue with our chosen enzyme C23O was that we weren’t sure whether it could be successfully expressed in B.subtilis as most papers referred to assays within an E.coli host. However it was later found to have been expressed successfully in B. subtilis by Zukowski et al. | + | An issue with our chosen enzyme C23O was that we weren’t sure whether it could be successfully expressed in B.subtilis as most papers referred to assays within an ''E.coli'' host. However it was later found to have been expressed successfully in B. subtilis by Zukowski ''et al''. |
| + | |||
| + | |||
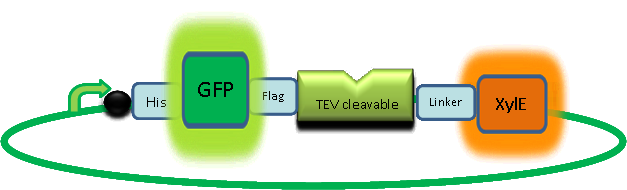
| + | Here's a picture of the final construct: | ||
| + | |||
| + | [[Image:IC_Module3.JPG]] | ||
==References== | ==References== | ||
[1]An archetypical extradiol-cleaving catecholic dioxygenase: the crystal structure of catechol 2,3-dioxygenase (metapyrocatechase) from Pseudomonas putida mt-2 | [1]An archetypical extradiol-cleaving catecholic dioxygenase: the crystal structure of catechol 2,3-dioxygenase (metapyrocatechase) from Pseudomonas putida mt-2 | ||
Akiko Kita1, Shin-ichi Kita2, Ikuhide Fujisawa1, Koji Inaka3, Tetsuo Ishida4, Kihachiro Horiike4, Mitsuhiro Nozaki4 and Kunio Miki5, | Akiko Kita1, Shin-ichi Kita2, Ikuhide Fujisawa1, Koji Inaka3, Tetsuo Ishida4, Kihachiro Horiike4, Mitsuhiro Nozaki4 and Kunio Miki5, | ||
Revision as of 21:36, 19 October 2010
| Detection Module | Signaling Module | Fast Response Module |
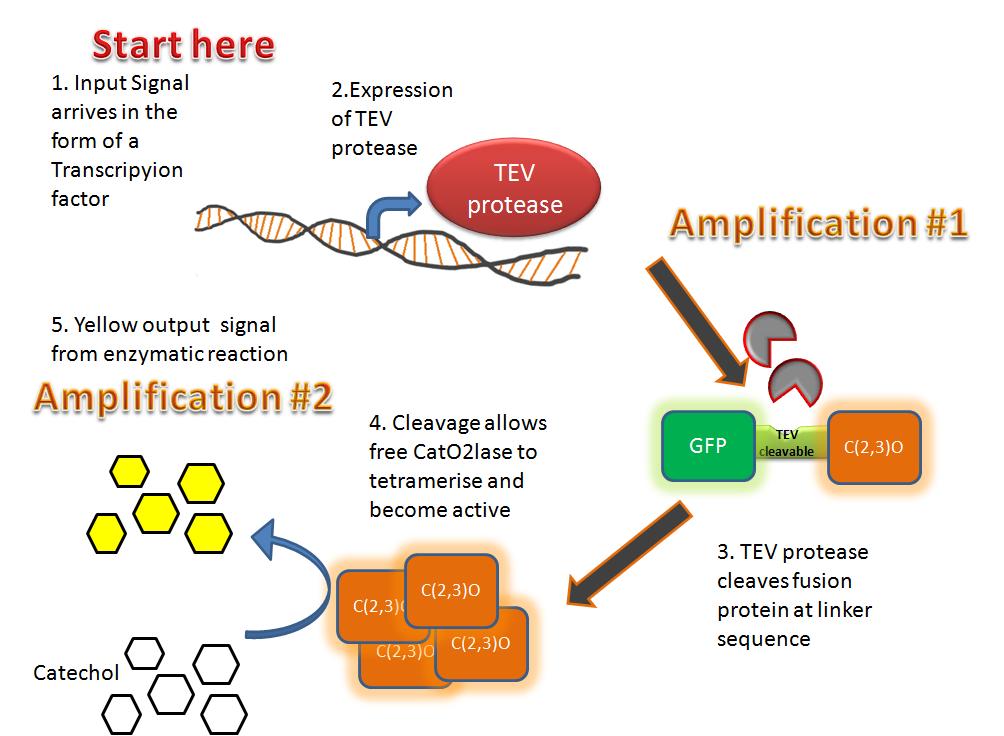
| We decided to design a new mechanism for parasite detection - by using the proteases they release. A novel protein bound to the cell surface, with a signaling peptide attached via a protease cleavage site. When the protease comes along, the signal peptide is released, allowing it to activate our signaling module. | To transduce the signal we used a quorum sensing system of a gram positive bacterium. The two component signal transduction system taken from S. pneumoniae transfers our peptide signal into the cell, activating the fast response module. | Our fast response mechanism is based around using two enzymatic amplification steps involving a transcripted enzyme, a deactivated enzyme and a presynthesised substrate. This greatly reduces the time required for producing a recognisable output, enabling useful field testing kits. |
| Fast Response Module | |
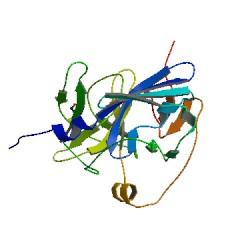
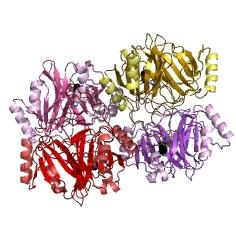
Overview of the output moduleTEVTEV proteaseTEV protease is a natural polyprotein cutting viral protease. Its expression in E.Coli (and consequently B .subtilis) is made efficient by codon optimisation and inducing expression of additional tRNAs. This enzyme has been used extensively in protein engineering as it has a number of key qualities including a high specificity (ENYLFQG), and importantly for our system it is relatively tiny (242 aa) compared to other protease candidates available. Below is can be visualized as it has been deposited in the PDB with accession number 1Q31; Due to its small size it’s likely that transcription and translation of the gene will not introduce limiting kinetic factors within our fast response system. The promoter region before the TEV gene was re-engineered to be that of the ComC DE promoter region. This would respond to activated and dimerized ComCDE transcription factors; induced by a result of our system activation. The TEV gene would then be transcribed and translated. TEV would then act upon its pool of inactive substrate already present in the cell. This substrate was an inactive form of the chose output enzyme Catechol 2,3 dioxygenase. C23O is a homotetramer and its crystal structure has been solved. See Kita et al here it is viewed with the accession code 1MPY. For our fast response module we have taken advantage of the tetramerization feature which produces the functioning C230 enzyme; the active site forms as a result of intersubunit and interface interactions after oligomerization; We have made a fusion protein by adding GFP to the N- terminus, with the two connected by a linker cleavable by TEV protease. We hoped that this modified monomer would be unable to undergo oligomerisation until the TEV protease is activated. TEV would specifically cleave the linker between GFP and C23O, resulting in C23O monomers that are capable of oligomerising. Homotetramers would then be active and could catalyse the chromogenic reaction. The N-terminus was favoured over the C-terminus for modification because it is more accessible to the protease with it being positioned on the external surface of the monomer. However, it is further away from the oligomerisation interfaces and so might not actually prevent oligomerisation. The C-terminus, on the other hand, is less accessible, and by fusing GFP there it could prevent entry of the substrate into the funnel that runs through the enzyme. It is closer to the oligomerisation domain and so fusing GFP would be more likely to prevent oligomerisation. After observing C23O in Pymol, and in addition information obtained from Kita et al which shows that the projecting loop is needed for dimerization and that this is very near to the N-terminus, we chose to engineer (by straightforward DNA synthesis) GFP plus linker (GGGSGGGS ENYLFQG) onto the N-termini of our C230 monomers.
Image taken from ftp://ftp.genome.jp/pub/kegg Where catechol is the colourless substrate converted by ring cleavage into 2 hydroxymuconate semialdehyde. C230Catechol 2,3 dioxygenaseCatechol 2,3-dioxygenase (also known as C23O or MPC) catalyses the conversion of a colourless substrate (catechol or substituted catechols) into a bright yellow product (2-hydroxymuconic semialdehyde) within seconds of substrate addition. We performed our specific plate assays using a 100mM stock solution of catechol and via Pasteur pipette covering individual colonies in the solution. Liquid assays were performed with addition of a pre-made known concentration of catechol typically 0.05-0.35M diluted with DDH20. It was convenient that a Biobrick for the XylE gene was already present in the registry and we used this along with biobrick cloning methods to generate many of our XylE final and testing constructs. An issue with our chosen enzyme C23O was that we weren’t sure whether it could be successfully expressed in B.subtilis as most papers referred to assays within an E.coli host. However it was later found to have been expressed successfully in B. subtilis by Zukowski et al.
References[1]An archetypical extradiol-cleaving catecholic dioxygenase: the crystal structure of catechol 2,3-dioxygenase (metapyrocatechase) from Pseudomonas putida mt-2 Akiko Kita1, Shin-ichi Kita2, Ikuhide Fujisawa1, Koji Inaka3, Tetsuo Ishida4, Kihachiro Horiike4, Mitsuhiro Nozaki4 and Kunio Miki5, |
 "
"