in vivo study
Abstract
Gene therapy offers a great tool for treatment of various human diseases and has shown tremendous promise and success in many recent clinical trials. Nevertheless, two most critical hurdles that remain are 1) the development of delivery vectors that allow for the highly cell- and tissue-specific expression of a gene of interest (GOI), as well as 2) the implementation of novel systems that permit the tightly controlled and fine-tuned expression of this gene in human patients. To fill in these two essential gaps, the iGEM Team 2010 of Heidelberg is introducing molecular evolution of viral gene transfer vectors as well as mi(cro)RNA-based gene regulation technology into the realm of synthetic biology. By combining these two powerful new methodologies, we have succeeded in engineering synthetic viral miRNA vectors that we could demonstrate to provide potent, specific and regulated gene expression in cultured mouse and human hepatocytes. Most importantly, we also document the successful use of our novel approach to achieve tightly controlled and liver-specific expression of a vector-encoded recombinant DNA in intact livers of adult mice. Our work and data thus pave the way for the future further expansion and utilization of our unique miRNA/vector kits in numerous biotechnological and especially biomedical applications.
Introduction
Gene therapy offers a great tool for treatment of various diseases such as failure, muscular dystrophies, and cystic fibrosis (Zincarelli, Soltys et al. 2008).
The iGEM project of the Heidelberg team 2010 is dealing with tuning gene expression and directing target specificity.
Therefore AAV capsids are shuffled and under a stringent selection pressure the perfect tissue specific virus with a shuffled capsid gene should be picked. Even though AAVs are the perfect viruses in the context of gene therapy, as the vector is neither pathogenic nor toxic in cell culture and in vivo (Grimm, 2002) it is still not possible to selectively choose an AAV serotype which are tissue specific. It is for example known that a lot of rational modifications of AAV capsid genes (by inserting peptides or introducing some point mutations) may lead to viruses which are really efficient in cell culture but would never work in vivo. Therefore the capsid shuffling introduces a perfect method for constructing AAV capsid genes which can be shuffled in any combination of choice. (Grimm et al., 2008)
The possibility of targeting and even tuning expression in a certain tissue or cell line by using miRNA binding sites leads to even fine-tuned and more specific expression of the GOI. The binding sites can be either constructed by the miBS designer or by rational design. They can be validated using the miMeasure plasmid or the miRtuning construct. After having characterized the binding sites of choice a specific and tuned expression cassette can be introduced into a viral construct and infected into mice. And this is exactly what we did:
We rationally designed binding sites, cloned them into the miTuner kit and characterized them. We produced and own shuffled capsid library and after applying selection pressure one clone showed already very fast good infection efficiencies in culture. For this very reason we tested our shuffled capsid and also an AAV8 WT capsid packaging a Luciferase construct driven by the same promoter. This constructs tagged with different binding site combinations of choice in the 3’UTR were used for producing viral constructs for testing on- off and tuning properties of our system.
Results
Mice injection
The tail vein injection was chosen so as to assess AAV serotype tissue tropism;
the luciferase transgene was used for visualizing the relative vector
distribution in all the animals in a real-time manner. This allows for in vivo imaging and time lapse experiments.
Bioluminescence imaging

Discussion
Methods
Contructs
The in vivo analysis should enlighten our gene therapy approach using AAV tropism as well as miRNA binding sites as trigger for expression. The following constructs have been subcloned separately into the AAV context to accomplish those tasks:
- positive control,
- off-targeting construct,
- synthetic tuning construct and
- on-targeting construct.
All but one virus were packaged by the AAV rep and cap gene with Adenovirus 5 (Ad5) as a helper plasmid. Accordingly, one virus construct was packaged into a shuffled cap gene from our homology based capsid shuffling attempt.
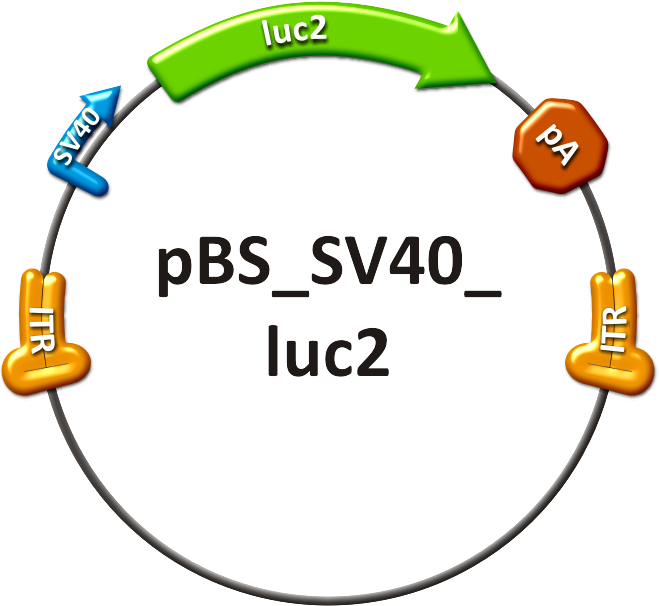
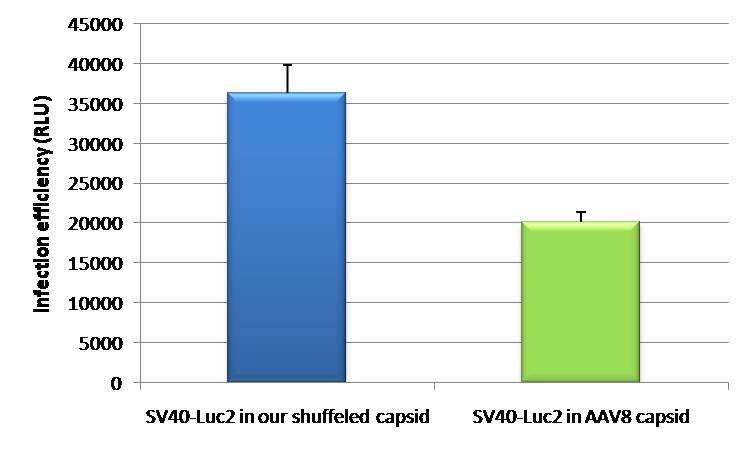
- The positive control (see sidebar, fig. 1) consisted of the SV40 promoter driving a firefly luciferase (luc2) gene, thereby leading to an unspecific expression of the luciferase protein in all mice tissues. In addition to packaging this construct into a wild type AAV virus, the positive control was also packaged as a transgene into our shuffled capsid which after random selection was already able to positively transduce Huh7 and HepG2 cells in vitro.
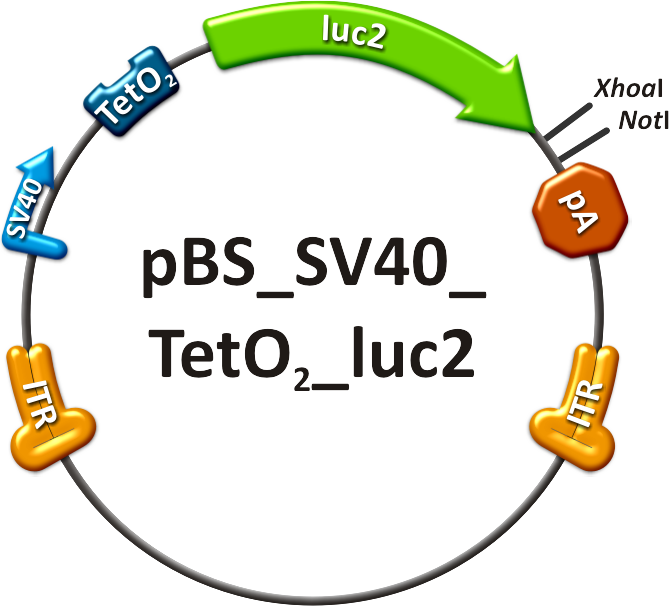
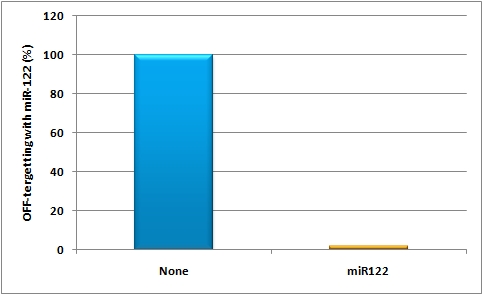
- The off-targeting construct (see sidebar, fig. 2) was composed of an SV40 promoter driving a firefly luciferase (luc2) gene with binding sites against miR-122 behind it. In order to achieve the highest expression in all mice cells but the liver cells - a single perfect binding site of miR-122 was used for in vivo study.
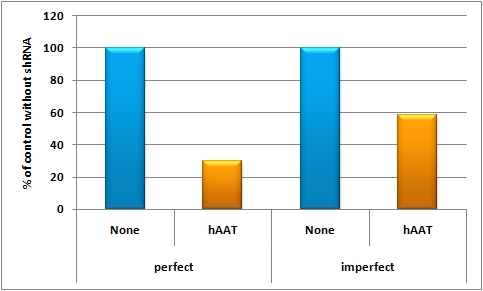
- The synthetic tuning construct (see sidebar, fig. 3) consisted of two viruses injected at the same time in the mice. The one virus packaged the expression construct of shRNA haat driven by the H1 promoter ("tuning" construct, see sidebar, fig.3). The second virus packaged the following transgene: SV40 promoter driving luc2 with shRNA haat binding site behind it ("tuned" construct, see sidebar, fig. 4). In order to ensure a synthetic tuning effect, a perfect binding site and one with a bulge that was introduced at position 9-12 were used for in vivo experiments, respectively. Those two binding sites should lead to a significant knockdown in the first case and a slight repression of luciferase expression in the latter as compared to the positive control.
- The on-targeting construct consisted of two independent viruses which were co-infected into mice, as well. One of these viruses packaged the Tet Repressor (TetR) driven by an SV40 promoter ("repressor" construct, see sidebar, fig. 5). The expression of TetR is under the control of miR-122 as four binding sites of this miRNA were cloned into the 3’UTR of the gene. The second virus was composed of an SV40 promoter driving the Tet operator (TetO2) which monitors the expression of luc2 ("operator" construct, see sidebar, fig. 6). With this setup, luc2 expression should be inhibited by the TetR in all mice tissues except for liver cells, where TetR is down-regulated by miRNA 122.
Production of recombinant virus
The viruses were produced in HEK 293-T cells and purified on an iodixanol gradient according to the virus production protocol.
Before infection, the titer of the viruses was quantified using quantitative realtime PCR.
Procedure involving animals
The mouse experiments were conducted in accordance with the animal facility of the German Cancer Research Center in Heidelberg. Female NMRI mice were obtained from a collaboration with Dr. Oliver Müller. At 8-10 weeks of age, the animals were injected in the tail vein (TV), with ~ 1x1011 particles of AAV-SV40-luciferase in 200µl of 1x phosphate-buffered saline. The mice are transferred to a holding device which restrains the mouse while allowing access to the tail vein. The tails were warmed before the injections and injections were carried out using 27 gauge needles. All the mice recoverd from the injection quickly without loss of mobility or interruption of grooming activity [http://2010.igem.org/Team:Heidelberg/Project/Mouse_Infection#References (Zincarelli et al., 2008)].
in vivo animal imaging
Mice were anesthesized in an isofluran chamber. The mice were injected intraperitoneally with 200µl of a 30 mg/ml concentration of D-luciferin. This injection starts the luminescence of luc2. Mice were measured for one to seven minutes post injection under the in vivo bioluminometer.
References
Grimm, D. (2002). "Production methods for gene transfer vectors based on adeno-associated virus serotypes." Methods 28(2): 146-157.
Grimm, D., J. S. Lee, et al. (2008). "In vitro and in vivo gene therapy vector evolution via multispecies interbreeding and retargeting of adeno-associated viruses." J Virol 82(12): 5887-5911.
Zincarelli, C., S. Soltys, et al. (2008). "Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection." Mol Ther 16(6): 1073-1080.

 "
"