Team:Heidelberg/Notebook/miMeasure/October
From 2010.igem.org
| (3 intermediate revisions not shown) | |||
| Line 1: | Line 1: | ||
| - | {{:Team:Heidelberg/ | + | {{:Team:Heidelberg/Single}} |
| - | {{:Team:Heidelberg/ | + | {{:Team:Heidelberg/Single_Pagetop|note_mimeasure}} |
| - | + | {{:Team:Heidelberg/Side_Top}} | |
| - | {{:Team:Heidelberg/ | + | |
{| cellpadding="5" cellspacing="0" style="text-align: center; color:#4e93a4; border: 3px solid #4e93a4;" | {| cellpadding="5" cellspacing="0" style="text-align: center; color:#4e93a4; border: 3px solid #4e93a4;" | ||
| Line 56: | Line 55: | ||
|- | |- | ||
|'''25'''||'''26'''||'''27'''||'''28'''||'''29'''||'''30'''||'''31''' | |'''25'''||'''26'''||'''27'''||'''28'''||'''29'''||'''30'''||'''31''' | ||
| - | |||
|} | |} | ||
| + | |||
| + | {{:Team:Heidelberg/Side_Bottom}} | ||
| + | |||
| + | |||
| + | __NOTOC__ | ||
| + | |||
| + | ===01/10/2010=== | ||
| + | <br /> | ||
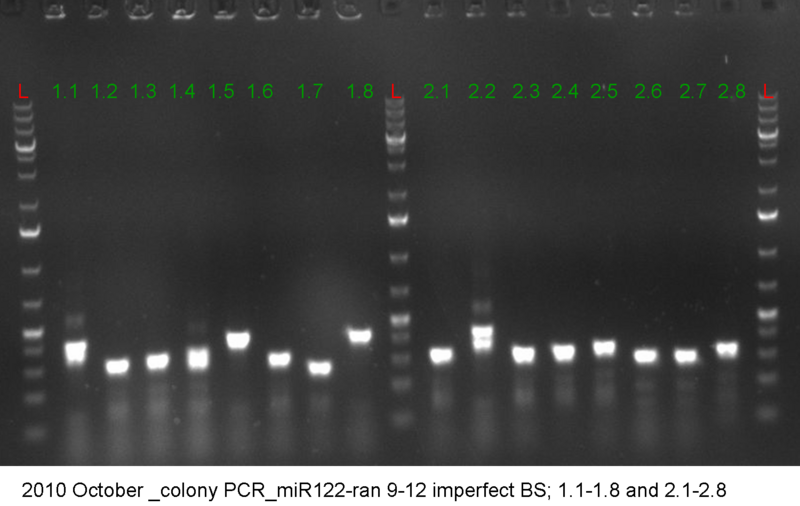
| + | [[Image:1.1-2.8_colony_PCR_miR122_9-12_ranomized.png|thumb|400 px|right]] | ||
| + | |||
| + | * reasonable number of colonies on each plate | ||
| + | * pick 8 colonies of each construct --> 32 colonies | ||
| + | * colony PCR | ||
| + | |||
| + | * 7 minipreps of promising candidates and sent for sequencing | ||
| + | |||
| + | |||
| + | ===02/10/2010=== | ||
| + | |||
| + | |||
| + | * digest pSB1C3 with DpnI, EcoRI, PstI | ||
| + | * digest pSB1A3 with DpnI, EcoRI, PstI | ||
| + | |||
| + | |||
| + | * digest 4x pSB1C3 (5 µL BSA, NEB buffer 2, 1 µL PstI, 1 µL EcoRI, 1 µL DpnI, 1µg construct = 4 µL construct, 33 µL H2O) | ||
| + | * digest 4x pSB1A3 (5 µL BSA, NEB buffer 2, 1 µL PstI, 1 µL EcoRI, 1 µL DpnI, 1µg construct = 4 µL construct, 33 µL H2O) | ||
| + | |||
| + | |||
| + | |||
| + | ===03/10/2010=== | ||
| + | |||
| + | Ligation of miR221, 1179 and 4286 raPCRs to pSB1C3 backbone (each 0S, 0L, 10S, 10L) using quick ligase and plate to chloramp. LB-plates | ||
| + | |||
| + | <br /><br /><br /> | ||
| + | |||
| + | |||
| + | ===04/10/2010=== | ||
| + | |||
| + | colony PCRs of the miR221, 1179 and 4286 did not show any nice results. | ||
| + | Set up 20 minipreps anyway | ||
| + | <br /><br /><br /> | ||
| + | |||
| + | |||
| + | ===05/10/2010=== | ||
| + | |||
| + | * digest 1µg of all binding sites (1A, 3F, 1_3, 1_5, 1_7, 1_7, 3_7) with: | ||
| + | ::* EcoRI | ||
| + | ::* PstI | ||
| + | ::* buffer2 | ||
| + | ::* BSA | ||
| + | to ligate in miMeasure | ||
| + | |||
| + | |||
| + | <br /><br /><br /> | ||
| + | |||
| + | |||
| + | ===06/10/2010=== | ||
| + | Restriction digest of successful sequenced miRBS for miR122ran, 221, 1179 (they are in pSB1C3 backbone). 500ng with 1 µL EcoRI/PstI | ||
| + | |||
| + | Set up Minipreps for miRBS for miR122 and miMeasure construct (6 ml LB) in the morning. Miniprep in the evening. | ||
| + | |||
| + | Those Minipreps were transfected to HUH7, HEPG2 and Hepa 1.6 cells using fugene transfectand (see Measurement notebook and figure below). | ||
| + | |||
| + | raPCR of miR320b has not shown a nice smear. Will be trashed and repeated. | ||
| + | |||
| + | |||
| + | * 20 Minis | ||
| + | * tranfect cells | ||
| + | |||
| + | |||
| + | ===07/10/2010=== | ||
| + | Ligation of succesful sequenced miRBS of miR122ran, 221, 1179 (10 in total) | ||
| + | |||
| + | <br /><br /><br /> | ||
| + | |||
| + | ===13/10/2010=== | ||
| + | * digest miMeasure with PstI and EcoRI | ||
| + | * digest 16 different raPCRs (8x miR230b, 8x 4286) | ||
| + | * ligate 16 binding site patterns into miMeasure | ||
| + | * transform newly ligated constructs | ||
| + | <br /><br /><br /> | ||
| + | |||
| + | |||
| + | ===14/10/2010=== | ||
| + | * a reasonable | ||
| + | * Screening of 40 colonies of miR4286 (colony PCR) | ||
| + | * Screening of 32 colonies of miR320b (colony PCR) | ||
| + | |||
| + | <br /><br /><br /> | ||
| + | |||
| + | |||
| + | |||
| - | {{:Team:Heidelberg/ | + | {{:Team:Heidelberg/Single_Bottom}} |
Latest revision as of 00:58, 27 October 2010

01/10/2010
02/10/2010
03/10/2010Ligation of miR221, 1179 and 4286 raPCRs to pSB1C3 backbone (each 0S, 0L, 10S, 10L) using quick ligase and plate to chloramp. LB-plates
04/10/2010colony PCRs of the miR221, 1179 and 4286 did not show any nice results.
Set up 20 minipreps anyway
05/10/2010
to ligate in miMeasure
06/10/2010Restriction digest of successful sequenced miRBS for miR122ran, 221, 1179 (they are in pSB1C3 backbone). 500ng with 1 µL EcoRI/PstI Set up Minipreps for miRBS for miR122 and miMeasure construct (6 ml LB) in the morning. Miniprep in the evening. Those Minipreps were transfected to HUH7, HEPG2 and Hepa 1.6 cells using fugene transfectand (see Measurement notebook and figure below). raPCR of miR320b has not shown a nice smear. Will be trashed and repeated.
07/10/2010Ligation of succesful sequenced miRBS of miR122ran, 221, 1179 (10 in total)
13/10/2010
14/10/2010
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 "
"