Team:Freiburg Bioware/NoteBook/Labjournal/Cellculture
From 2010.igem.org
(→Cellculture) |
(→Cellculture) |
||
| Line 31: | Line 31: | ||
==Cellculture== | ==Cellculture== | ||
| - | This subpage is for cellculture | + | This subpage is for cellculture, the structure of each experiment is following: |
===<p style="font-size:17px; background-color:#00dd77;">Date of first action, title of the experiment</p>=== | ===<p style="font-size:17px; background-color:#00dd77;">Date of first action, title of the experiment</p>=== | ||
| Line 41: | Line 41: | ||
In this section, the design of the experiment is explained. | In this section, the design of the experiment is explained. | ||
<br /> | <br /> | ||
| - | |||
<b>The results</b><br /> | <b>The results</b><br /> | ||
Interpretation and discussion of our results. | Interpretation and discussion of our results. | ||
Revision as of 10:08, 9 October 2010
Team:Freiburg Bioware/SubNoteBook
- March (labday 1)
- April (labday 2 - 5)
- May (labday 6 - 17)
- June (labday 18 - 45)
- July (labday 46 - 75)
- August part 1 (labday 76 - 92)
- August part 2 (labday 93 - 106)
- September part 1 (labday 107 - 123)
- September part 2 (labday 124 - 135)
- October part 1 (labday 136 - 145 )
- October part 2 (labday 146 - 155 )
- October part 3 (labday 156 - 166 )
- November (labday 167 - 170 )
- Cellculture
Cellculture
This subpage is for cellculture, the structure of each experiment is following:
Date of first action, title of the experiment
The motivation
This short introduction provides an inside into our thoughts for this experiment.
The plan
In this section, the design of the experiment is explained.
The results
Interpretation and discussion of our results.
11.8.2010 Transduction of HT1080 with no AAV, AAV without GOI and AAV with 10µg -> FACS
The motivation: In the past, the percentage of living cells in FACS was very low (~ 70%) so we want to check if the AAV-induced stress is responsible for cellular death, or the detaching procedure (trypsin and mechanical stress).
The plan
Plate I
| control, no cells | no GOI 150µl | 10µg 500µl |
| control, no virus | no GOI 300µl | 10µg 750µl |
Plate II
| control, no cells | no GOI 150µl | 10µg 500µl |
| control, no virus | no GOI 300µl | 10µg 750µl |
Plate III
| control, no cells | no GOI 150µl | 10µg 500µl |
| control, no virus | no virus | 10µg 750µl |
The results
ADD FACS DATA
The YFP expression is still quite low. This was kind of anticipated, the success in this experiment is the fact, that the amount of living cells is at a very high level! (~90%). The conclusion: Detach the cells fast! Use PBS which is not at 37°C (the PBS was 25min in the water bath), transfer the cells fast on ice after detaching!
The next steps are:
- Is it possible, that freeze/thaw circles reduce the transduction efficiency?
- Why is the YFP-expression that low? (~28-30%)
12.8 Virus production, seeding A431 cells
The motivation
In first place we want to create new stock of AAV without GOI, the next viral stock is with correct TK/GMK, the last stocks are with different YFP-amounts to check if the amount of GOI is a critical step for transduction effency (transgene expression).
The plan
See transduction protocoll for further information, the final cantrifugation step was done with 2000 g 5 min.
The results
Ten viral stocks with 3 ml each were prepared.
| Plate | pAAV_RC/µg | pHelper/µg | GOI/µg |
| 1 | 3,3 | 3,3 | no GOI |
| 2 | 6,6 | 3,3 | no GOI |
| 3 | 3,3 | 3,3 | 3,3 TK_GMK clone1 |
| 4 | 3,3 | 3,3 | 3,3 TK_GMK clone1 |
| 5 | 3,3 | 3,3 | 3,3 TK_GMK clone2 |
| 6 | 3,3 | 3,3 | 3,3 YFP |
| 7 | 3,3 | 3,3 | 10 YFP |
| 8 | 3,3 | 3,3 | 20 YFP |
| 9 | 3,3 | 3,3 | 40 YFP |
| 10 | 3,3 | 3,3 | 60 YFP |
The nomenclature of the plasmids is wrong, the date is 12.8. not 13.8
13.8 Seeding HT1080 for transduction at saturday, Seeding HEK293 for transfection
The motivation
The plan
Platte 1:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 1 | 300µl virus 2 |
| B | control, no virus | 600µl virus 1 | 600µl virus 2 |
Platte 2:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 3 | 300µl virus 4 |
| B | control, no virus | 600µl virus 3 | 600µl virus 4 |
Platte 3:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 5 | 300µl virus 6 |
| B | control, no virus | 600µl virus 5 | 600µl virus 6 |
Platte 4:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 7 | 300µl virus 8 |
| B | control, no virus | 600µl virus 7 | 600µl virus 8 |
Platte 5:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 9 | 300µl virus 10 |
| B | control, no virus | 600µl virus 9 | 600µl virus 10 |
Platte 6:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 3 | 300µl virus 4 |
| B | control, no virus | 600µl virus 3 | 600µl virus 4 |
Platte 7:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 3 | 300µl virus 4 |
| B | control, no virus | 500µl virus 3 | 500µl virus 4 |
Platte 8:
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus 5 | 300µl virus 5 |
| B | control, no virus | 600µl virus 5 | 600µl virus 5 |
The results
16.8: The fluorescence was quite low (~ 5%)in nearly every well! The 40µg YFP wells were the only exception.
The conclusions
The incubation step of CaCl2 seems to be crucial! The next transfection protocol is with a 30 min incubation on ice step!
Seeding HEK293 for transfection at monday 16.8
The motivation
Which confluence of HEK293 cells is optimal for AAV production?
The plan
We seed different amounts of cells, and check the highest titer.
| Plate | amount of cells |
| 1 | 100 000 |
| 2 | 200 000 |
| 3 | 400 000 |
| 4 | 500 000 |
| 5 | 800 000 |
| 6 | 1 000 000 |
| 7 | 1 200 000 |
| 8 | 1 500 000 |
| 9 | 1 750 000 |
| 10 | 1 750 000 not sure |
The results
Fluorescence microscopy of transduction at 14.8 transfection of plates from 13.8
Working steps:
- Remove the three plasmids to be co-transfected (the recombinant pAAV expression plasmid or control plasmid, pAAV-RC, and pHelper) from storage at –20°C.
- Pipet XXX µg of each of the three plasmid DNA solutions into an eppi. The standart protocol is: 10 µg per plasmid, experiment data showed that the amounts (3,3 µg, 6,6 µg and 10 µg per plasmid) showed similar results
- Fill up to 300 µl with sterile water. Add 300µl of 0.5 M CaCl2 and mix gentlyIncubation step 45 min on ice !!!!!!
- Pipet 600 µl ml of 2 × HBS into a 15-ml falcon.
- Vortex the falcon (with the 2x HBS) gently!!!! while pipetting the DNA/CaCl2 solution dropwise into the falcon (use the Pipetus).
- Apply the DNA/CaCl2/HBS suspension to the plate of cells in a dropwise fashion(experimental data shows that resuspending-fashion is better for high titer), swirling gently to distribute the DNA suspension evenly in the medium.
- Return the tissue culture plate to the 37°C room for 6 hours.
- At the end of the incubation period, remove the medium from the plate and wash cells with warm PBS, replace it with 10 ml of fresh DMEM growth medium.This is a crucial step! The transfection-stress kills nearly all cells if theres no medium change
- Return the plate to the 37°C incubator for an additional 72 hours.
Seeding HT1080
The motivation
We want to check if the amount of HT cells is crucial for Transduction (The second transduction was daone with 30.000 cells, and had a high YFP expression)
The plan
3* 6er dishes with 100.000 cells and 2* 6er dishes with 50.000 cells were seeded.
The results
FILL IN PICTURES
Seeding AAV293
The motivation
We want to create new viral stocks.
The plan
15 * 10 cm2 with 2.000.000 cells on each plate were created
17.8 Transduction of plates with different amounts of cells
The plan
Platte 1 YFP: 50.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus | 300µl virus |
| B | control, no virus | 300µl virus | 300µl virus |
Platte 2 YFP: 50.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus | 300µl virus |
| B | control, no virus | 300µl virus | 300µl virus |
Platte 2 YFP: 50.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus | 300µl virus |
| B | control, no virus | 300µl virus | 300µl virus |
Platte 3 YFP: 100.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus | 300µl virus |
| B | control, no virus | 300µl virus | 300µl virus |
Platte 4 YFP: 100.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus | 300µl virus |
| B | control, no virus | 300µl virus | 300µl virus |
Platte 5 TKGMK: 100.000 cells per well
| 1 | 2 | 3 | |
|---|---|---|---|
| A | control, no cells | 300µl virus | 300µl virus |
| B | control, no virus | 300µl virus | 300µl virus |
18.8 Transfection
The Motivation
Transfection with different amounts of REP/CAP and pHELPER. Additionally a
The plan
Transfection of AAV293 cells
Investigator: Adrian, Kerstin
- 2x106 cells were seeded (instead of 3x106) --> hopefully better virus-production
- used plasmids:
- P50 c= 429 ng/µl (RC)
- P64 c= 500 ng/µl (pHelper)
- P229 c= 162 ng/µl (GOI=YFP)
- P228 c= 540 ng/µl (GOI=eGFP)
- P158a c= 1800ng/µl (RC mutant)
- P158a c= 1770ng/µl (RC mutant)
- 1. plate: Lipo-Transfection
- 2. plate: Lipo-Transfection
- 3. plate: 3,3 µg (RC), 3,3 µg (pHelper), 3,3 µg (YFP)
- 4. plate: 10 µg (RC), 10 µg (pHelper), 3,3 µg (YFP)
- 5. plate: 10 µg (RC mutant P158a), 10 µg (pHelper), 3,3 µg (YFP)
- 6. plate: 10 µg (RC mutant P158b), 10 µg (pHelper), 3,3 µg (YFP)
- 7. plate: 3,3 µg (RC mutant P158a), 3,3 µg (pHelper), 3,3 µg (YFP)
- 8. plate: 3,3 µg (RC mutant P158b), 3,3 µg (pHelper), 3,3 µg (YFP)
- 9. plate: 10 µg (RC), 10 µg (pHelper), 3,3 µg (eGFP)
- 10. plate: 10 µg (RC), 10 µg (pHelper), 10 µg (eGFP)
- 11. plate: 10 µg (RC), 10 µg (pHelper), 20 µg (eGFP)
- 12. plate: 10 µg (RC), 10 µg (pHelper), 40 µg (eGFP)
20.8
The Motivation
Seeding HT1080 for transfection with viral stocks from 18.8
The Plan
We harvest four T75 flasks and seed them into 6er dishes (200.000 cells in each well)
the Results
The Facs data at 22.8
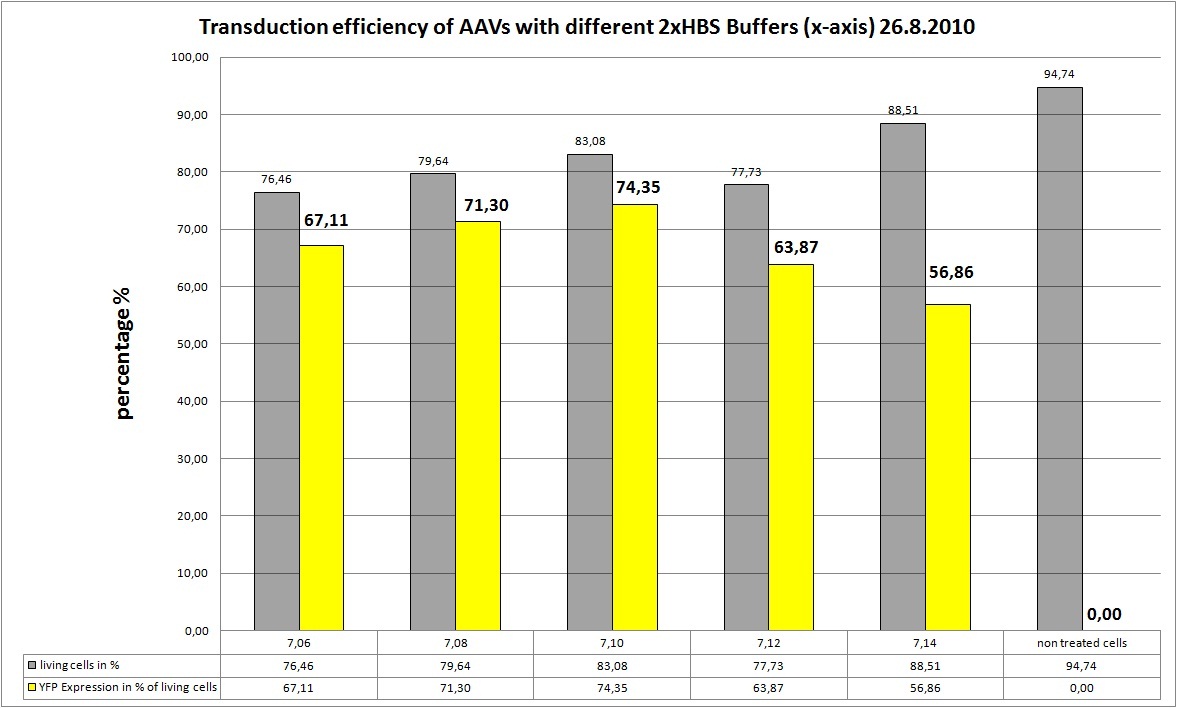
21.8 Fluorescence microscopy, Transfection, AAV-Harvesting and Transduction
Transfection with different HBS-Buffers and with stratagene standard protocol
The Motivation
Transfection of AAV293 cells from 19.8 (ten 10cm2 dishes with 2.000.000 cells each) with original standard protocoll!!! We want to try different 2xHBS Buffers (pH) and their influence on transfection efficiency!
The Plan
Requiered goods: 0,5 M CaCl2 , 2x HBS-Buffer , autoclaved millipore water, falcons, eppis, sven's vortexer sterilized with EtOH
Working steps:
- Remove the three plasmids to be co-transfected (the recombinant pAAV expression plasmid or control plasmid, pAAV-RC, and pHelper) from storage at –20°C.
- Pipet 10 µg of each of the three plasmid DNA solutions into an eppi.
- Fill up to 300 µl with sterile water. Add 300µl of 0.5 M CaCl2 and mix gently
- Pipet 600 µl ml of 2 × HBS into a 15-ml falcon.
- Vortex the falcon (with the 2x HBS) gently!!!! while pipetting the DNA/CaCl2 solution dropwise into the falcon (use the Pipetus).
- Apply the DNA/CaCl2/HBS suspension to the plate of cells in a dropwise fashion, swirling gently to distribute the DNA suspension evenly in the medium.
- Return the tissue culture plate to the 37°C room for 6 hours.
- At the end of the incubation period, remove the medium from the plate and wash cells with warm PBS, replace it with 10 ml of fresh DMEM growth medium.
- Return the plate to the 37°C incubator for an additional 72 hours.
We try 5 different 2xHBS buffers:
- pH: 7.06
- pH: 7.08
- pH: 7.10
- pH: 7.12
- pH: 7.14
The transfected cells were seeded at 19.8 (2.000.000 cells per 10 cm2)
Used Plasmids: Standart protocol: 10µg of each plasmid
- GOI: mVENUS => P262, conc:1148ng/µl; used amount: 8,71µl
- GOI: TKGMK => P82b, conc:1500ng/µl; used amount: 6,67µl
- pHELPER => P64, conc:503ng/µl; used amount: 20µl
- RepCap => 10.8: 1348ng/µl; used amount: 7,41µl
- Remove the three plasmids to be co-transfected (the recombinant pAAV expression plasmid or control plasmid, pAAV-RC, and pHelper) from storage at –20°C.
- Pipet 10 µg of each of the three plasmid DNA solutions into an eppi.
- Add 1000µl of 0.3 M CaCl2 and mix gently
- Pipet 1000 µl ml of 2 × HBS into a 15-ml falcon.
- Pipett the DNA/CaCl2 solution dropwise into the falcon (use the Pipetus).
- Apply the DNA/CaCl2/HBS suspension to the plate of cells in a dropwise fashion, swirling gently to distribute the DNA suspension evenly in the medium.
- Return the tissue culture plate to the 37°C room for 6 hours.
- At the end of the incubation period, remove the medium from the plate and wash cells with warm PBS, replace it with 10 ml of fresh DMEM growth medium.
- Return the plate to the 37°C incubator for an additional 72 hours.
Eight new viral stocks were made with pHelper:20µl and RepCap:7,41µl each:
- mVenus:8,71µl; pH of 2xHBS:7,06
- mVenus:8,71µl; pH of 2xHBS:7,08
- mVenus:8,71µl; pH of 2xHBS:7,10
- mVenus:8,71µl; pH of 2xHBS:7,12
- mVenus:8,71µl; pH of 2xHBS:7,14
- TKGMK:6,67µl; pH of 2xHBS:7,08
- TKGMK:6,67µl; pH of 2xHBS:7,10
- TKGMK:6,67µl; pH of 2xHBS:7,12
the Results
AAV-Harvesting is at 24.8, same day Transduction and FACS will be done at 26.8
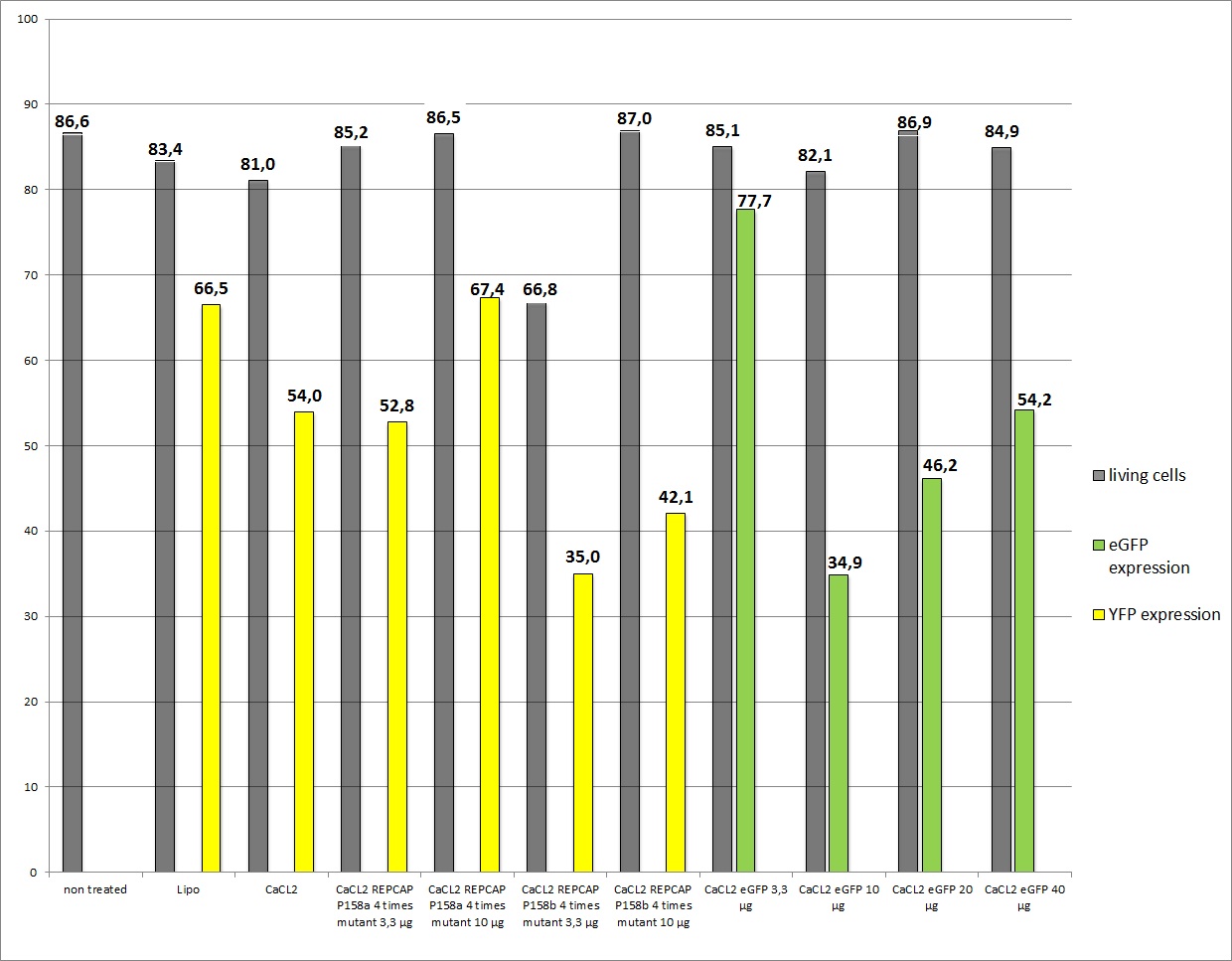
Harvesting viral stock from 18.8.2010 and following transduction
the motivation
We want to check the efficiency of Lipo-transfection and standard transfection with different ratios of REPCAP/pHelper, additionally if the modified RC (p158a and p158b) is still functionally.
The plan
Harvesting the cells with the Stratagene-standard protocoll.
The results
13 virals stocks were created (in 2.000.000 cells per 10cm2 dish):
- Lipo-Transfection 1 (2µl pHelper + 0,8µl RC + 6,2 µl YFP)
- Lipo-Transfection 2 (2µl pHelper + 0,8µl RC + 6,2 µl YFP)
- 3,3 µg of RC, pHelper and mVenus
- 10 µg RC, 10 µg pHelper, 3,3 µg GOI
- 10µg RC P158a four times mutant, 10µg pHelper, 3,3 µg mVenus
- 10µg RC P158b four times mutant, 10µg pHelper, 3,3 µg mVenus
- 3,3µg RC P158a four times mutant, 3,3µg pHelper, 3,3 µg mVenus
- 3,3µg RC P158b four times mutant, 3,3µg pHelper, 3,3 µg mVenus (negative control)
- 10µg RC, 10µg pHelper, 3,3µg GOI (broken arrow)
- 10µg RC, 10µg pHelper, 10µg GOI (broken arrow)
- 10µg RC, 10µg pHelper, 20µg GOI (broken arrow)
- 10µg RC, 10µg pHelper, 40µg GOI (broken arrow)
- 3,3 µg RC, 3,3 µg pHelper, 3,3 µg GOI
- negative control
- negative control
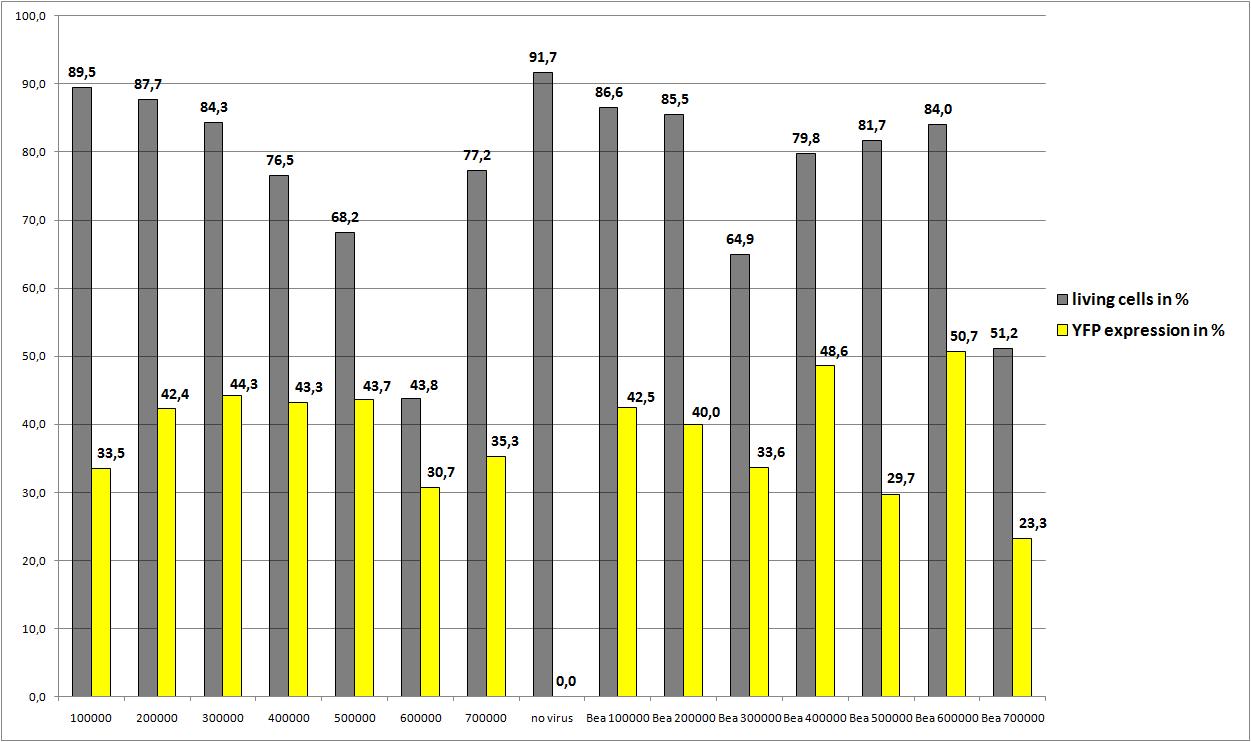
Checking transduction efficiency via fluorescence microscopy
the motivation
The transduction efficiency from the viral stocks which were created with different amounts of AAV293 was checked.
The plan
- plate 1:
- stock 1 (with 100.000 cells)
- stock 2 (with 200.000 cells)
- plate 2:
- stock 3 (with 400.000 cells)
- stock 4 (with 500.000 cells)
- plate 3:
- stock 5 (with 800.000 cells)
- stock 6 (with 1.000.000 cells)
- plate 4:
- stock 7 (with 1.200.000 cells)
- stock 8 (with 1.500.000 cells)
- plate 5:
- stock 9 (with 1.750.000 cells)
- stock 10 (with 1.750.000 cells)
The results
the optimal confluence of AAv293 cells is between 100 000 and 800 000 cells. This will be the field of investigation in the next experiments
25.8: Transfection of AAV293 with different amounts of cells, and two plasmid concentrations
The motivation
We want to define the final AAV293-concentration for optimal AAV-Production
The plan
- 100.000
- 100.000
- 200.000
- 200.000
- 300.000
- 300.000
- 400.000
- 400.000
- 500.000
- 500.000
- 600.000
- 600.000
- 700.000
- 700.000
10µg RC P158a four times mutant, 10µg pHelper, 3,3 µg mVenus P162 were pipetted on plates: 2, 4, 6, 8, 10 , 12, 14
3,3µg RC P158a four times mutant, 3,3µg pHelper, 3,3 µg mVenus P262 were pipetted on plates: 1, 3, 5, 7, 9, 11, 13
| Days | 22.8 | 23.8 | 24.8 | 25.8 | 26.8 | 27.8 | 28.8 | 29.8 | 30.8 |
|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | - | Seeding HT | Harvest + Transduction | - | FACS |
Data were not reliable (The amount of Goi (3,3 µg instead of 10µg) was not sufficient enough)so the average YFP expression is quite low, but the remarkable note is: Beas construct (pSB1C3_leftITR_CMV_betaglobin_mVenus_hGH_rightITR) fusioned from the created Biobricks still works!
Seeding AAV293 for 10xTransfection
The motivation
We want to create ten hypothetical identical viral stocks to estimate the standard deviation.
The plan
| Days | 30.8 | 31.8 | 1.9 | 2.9 | 3.9 | 4.9 | 5.9 | 6.9 |
|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | Seeding HT | Harvest + Transduction | FACS |
Transfection will be done with:
- RepCap:P357 10µg =>conc.:1274,8ng/µl used amount: 7,84µl
- pHelper:P356 10µg =>conc.:1068ng/µl used amount: 9,36µl
- mVenus:P261 => excel sheet P263: 10µg =>conc.:979ng/µl used amount: 10,21 µl
0,5 ml of each stock become deepfreeze in.
One stock will be completely used for Transduction 9,5 ml (rest of the stock) * 0,5 ml (amount per transduction) => 19 values for estimating standard derivation.
getting valide data of 10µg samples
The motivation
we want to check the YFP expression
The plan
| Days | 30.8 | 31.8 | 1.9 | 2.9 |
|---|---|---|---|---|
| Action | seeding HT | Transduction | - | FACS |
One stock will be completely used for Transduction 9,5 ml (rest of the stock) * 0,5 ml (amount per transduction) => 19 values for estimating standard derivation.
the results
28.8 Checking functionality of RC vectors
| Days | 28.8 | 29.8 | 30.8 | 31.9 | 1.9 | 2.9 | 3.9 | 4.9 | 5.9 | 6.9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | - | Transfection | - | - | Harvest+Seeding HT | Transduction | - | FACS |
Transfection was done with:
- RepCap:P326 => 2481 ng/µl used amount: 4µl => 10µg
- RepCap:P325 => 2051 ng/µl used amount: 4,87µl => 10µg
- pHelper:P356 => 684 ng/µl used amount: 414,62µl => 10µg
- mVenus:P262 => 1148 ng/µl used amount: 8,7µl => 10µg
The results
Transfection will be done with:
- RepCap:P357 10µg =>conc.:1274,8ng/µl used amount: 7,84µl
- pHelper:P356 10µg =>conc.:1068ng/µl used amount: 9,36µl
- mVenus:P261 => excel sheet P263: 10µg =>conc.:979ng/µl used amount: 10,21 µl
4.9 checking constructs (GOIs) with/without HGH and beta globin and reassembled as control
The motivation
We want to check if these reassmbled constructs (GOIs) still work!
- P269 lITR_CMV_beta-globin_mVenus_HGH_rITR = 325 ng/µl => 9,66 µl
- P270 lITR_CMV_beta-globin_mVenus_HGH_rITR = 561 ng/µl => 7,26 µl
- P377 lITR_CMV_mVenus_HGH_rITR = 325,04 ng/µl => 30,76 µl
- P378 lITR_CMV_beta-globin_mVenus_rITR = 561 ng/µl => 17,8µl
All constructs are completed with p50 R/C =>> 26,45 µl and pHELPER 14,61µl
The plan
| Days | 4.9 | 6.9 | 7.9 | 8.9 | 9.9 | 10.9 | 11.9 | 12.9 |
|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | Seeding HT | Harvest + Transduction | FACS |
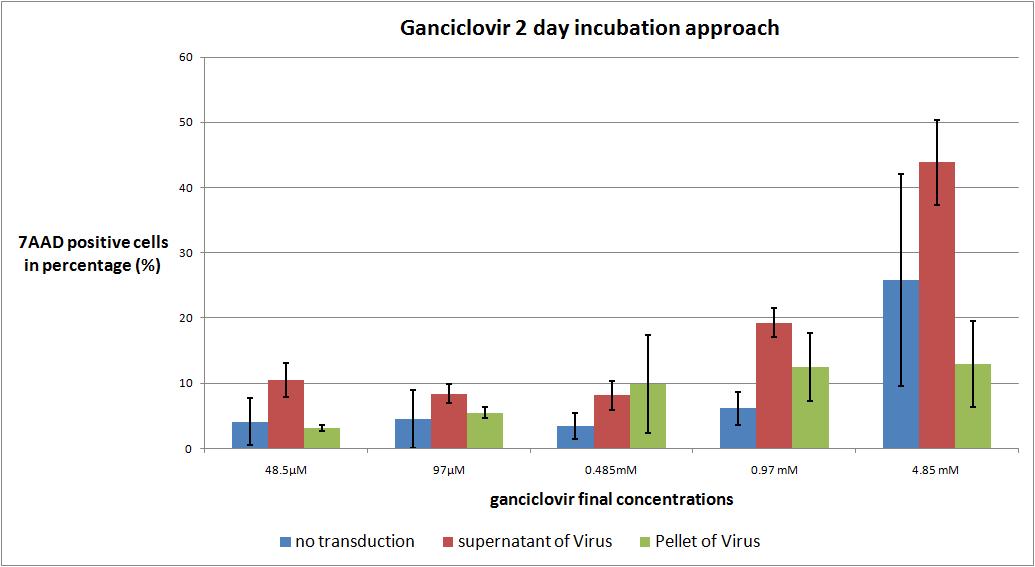
6.9 checking five ganciclovir concentrations for two day incubation approach optimal medication
The motivation
we want to check the optimal ganciclovir concentration, we use the TK/GMK Stocks (10 µg from each plasmid) from 24.8 with Buffer pH 7,10 and the pH12 stock.
The plan
| Days | 3.9 | 4.9 | 5.9 | 6.9 |
|---|---|---|---|---|
| Action | seeding HT | Transduction | - | FACS |
We want to use five different ganciclovir concentrations (the concentrations are absolute to the volume in the wells):
- 48.5µM
- 97 µM
- 0.485 mM
- 0.97 mM
- 4.85 mM
22.9 Transfection with Volkers R/C constructs
The motivation
We want to check if these R/C still work!
The plan
| Days | 20.9 | 21.9 | 22.9 | 23.9 | 24.9 | 25.9 | 26.9 | 27.9 | 28.9 |
|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293: 4*6wells | - | Transfection: 4 constructs | - | - | Seeding HT | Harvest + Transduction | - | FACS |
The results on the 23.9 it was shure that something went wrong with the nomenclature, so this approach is definitely not working => threwn away!
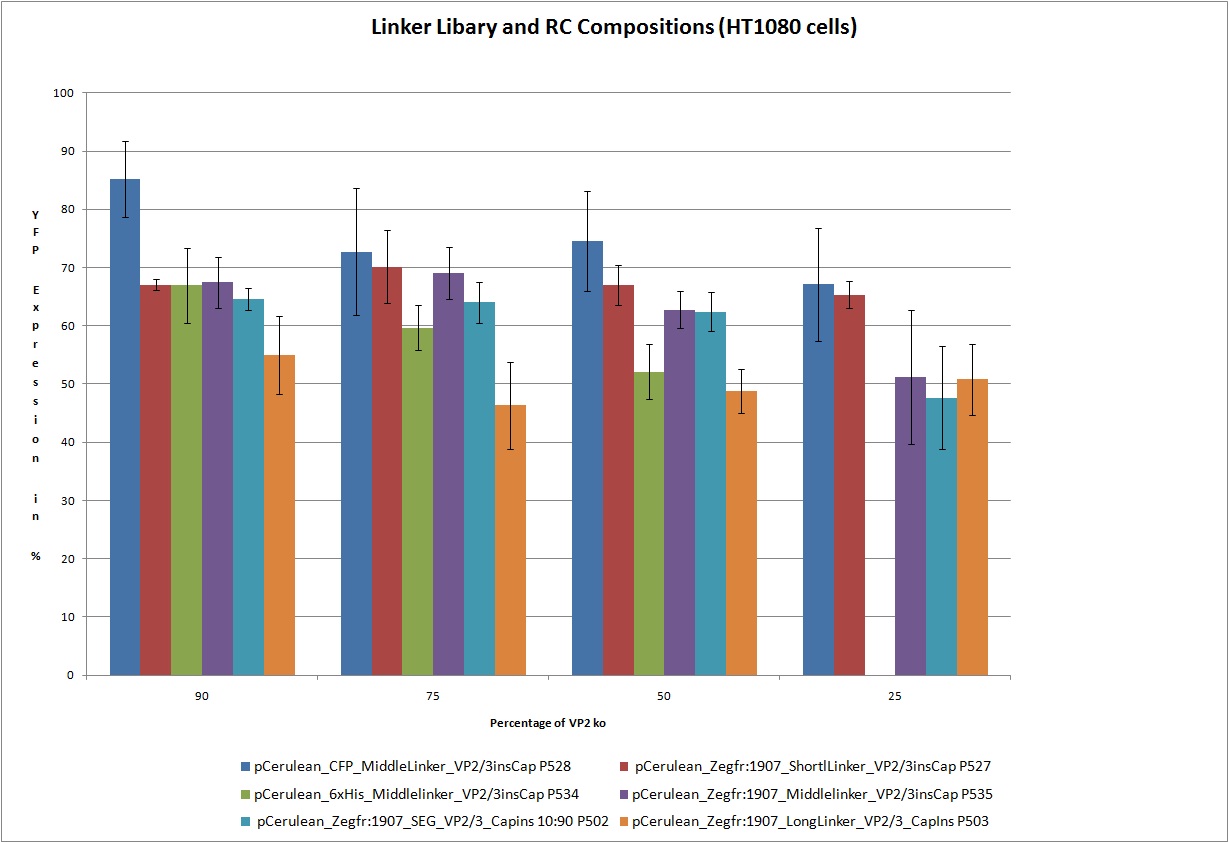
22.9 Testing of Hannas 6 VP-constructs with different compositions (10%, 25%, 50% and 75% fractions) with a VP2-knockout construct
The motivation
Test the 18 constructs for functionality on A431 and HT1080 cells.
The plan
| Days | 20.9 | 21.9 | 22.9 | 23.9 | 24.9 | 25.9 | 26.9 | 27.9 | 28.9 |
|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | - | Seeding HT | Harvest + Transduction | - | FACS |
22.9 Testing pTERT_mVENUS in R/C P326 and P431
The motivation
We want to check if the pTERT-promoter is realiable for tumor specific gene expression.
The plan
| Days | 22.9 | 23.9 | 24.9 | 25.9 | 26.9 | 27.9 | 28.9 | 29.9 | 30.9 | 1.10 | 2.10 | 3.10 | 4.10 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | - | Seeding HT | Harvest | - | - | Seeding HT, A293 and A431 | Transduction | FACS |
The results
28.9 Testing Loop Insertions
The motivation
The plan
| Days | 28.9 | 29.9 | 30.9 | 1.10 | 2.10 | 3.10 | 4.10 | 5.10 | 6.10 |
|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | - | Seeding HT | Harvest + Transduction | - | FACS |
The results
Experiments
The motivation
The plan
| Days | 22.9 | 23.9 | 24.9 | 25.9 | 26.9 | 27.9 | 28.9 | 29.9 | 30.9 |
|---|---|---|---|---|---|---|---|---|---|
| Action | seeding AAV293 | - | Transfection | - | - | Seeding HT | Harvest + Transduction | - | FACS |
The results
Citations
[1] Media:Freiburg10_Stratagene_AAV_helper_free_manual.pdf
[2] [http://www.copewithcytokines.de/cope.cgi?key=A431 Titel einfügen]
[3] Read, A. P.; Strachan, T. (1999). "Chapter 18: Cancer Genetics". Human molecular genetics 2. New York: Wiley. ISBN 0-471-33061-2.
[4] [http://www.biomol.de/dateien/infos_nr778.gif Titel einfügen]
[5] Media:Freiburg10 Titration of AAV-2 particles via a novel capsid ELISA.pdf
 "
"