Team:Brown/Project/Light pattern/Logic design
From 2010.igem.org
(→Logic Design) |
|||
| (3 intermediate revisions not shown) | |||
| Line 2: | Line 2: | ||
=Light-Pattern Controlled Circuit= | =Light-Pattern Controlled Circuit= | ||
{{:Team:Brown/templates/Lpmenu}} | {{:Team:Brown/templates/Lpmenu}} | ||
| + | |||
==Logic Design== | ==Logic Design== | ||
Before we begin our discussion of the circuit logic, let us first review what must be accomplished by this circuit: | Before we begin our discussion of the circuit logic, let us first review what must be accomplished by this circuit: | ||
| Line 36: | Line 37: | ||
===AND Module=== | ===AND Module=== | ||
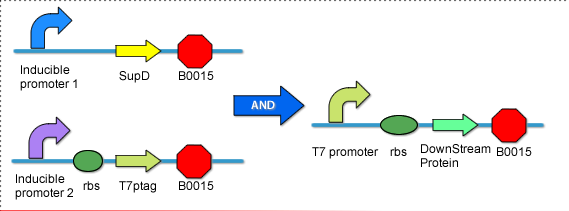
| - | This module regulates the expression/activation of the fourth and final state of the circuit. As with the third state, it is desired that the fourth state will only be triggered given prior activation of the previous states. Thus, an AND gate, where transcription is dependent upon the positive presence two transcription factors, is necessary to prevent the fourth state from occurring at the improper time. We looked at past team's attempts at AND gates, and found [https://2009.igem.org/Team:PKU_Beijing/Project/AND_Gate_1 PKU 2009's] description of the supD/T7ptag AND gate appropriate. | + | This module regulates the expression/activation of the fourth and final state of the circuit. As with the third state, it is desired that the fourth state will only be triggered given prior activation of the previous states. Thus, an AND gate, where transcription is dependent upon the positive presence two transcription factors, is necessary to prevent the fourth state from occurring at the improper time. We looked at past team's attempts at AND gates, and found [https://2009.igem.org/Team:PKU_Beijing/Project/AND_Gate_1 PKU 2009's] description of the supD/T7ptag AND gate appropriate. [[Image:AND_GATE.png|thumb|500px|center|The AND gate as outlined by team PKU '09. When both inducible promotors are active, supD tRNA and T7ptag form functional T7, inducing the T7 promoter.]] Last year, PKU achieved their optimal AND gate function using pSal and pBad. As we want to eliminate the need of multiple inputs, especially chemical ones, pSal was not a valid option for our circuit. Considering that we have already covered many of the available transcription factors in the registry, we decided to adopt the GAL4/UAS system. Gal4, a transcription factor derived from yeast, binds to the UAS (Upstream Activator Sequence) to induce transcription. By including GAL4 in state three, we can achieve activation of the AND gate following activation of the third state. |
==Putting it all together== | ==Putting it all together== | ||
| + | The diagram below is an interactive depiction of our circuit in its entirety. Press the LIGHT button to toggle the light ON and OFF. Note that when components are greyed out, they are "off" or repressed, and when they are black they are "on" or active. This depiction of the circuit was created using Adobe Flash CS4. | ||
| + | |||
| + | <html> | ||
| + | <center> | ||
| + | <embed src="https://static.igem.org/mediawiki/2010/d/dc/Lightpatterncircuit.swf" quality="high" bgcolor="#ffffff" width="800" height="600" name="mymoviename" align="" type="application/x-shockwave-flash" pluginspage="http://www.macromedia.com/go/getflashplayer"> | ||
| + | </center> | ||
| + | </html> | ||
Latest revision as of 23:12, 27 October 2010
Light-Pattern Controlled Circuit
Logic Design
Before we begin our discussion of the circuit logic, let us first review what must be accomplished by this circuit:
- Input
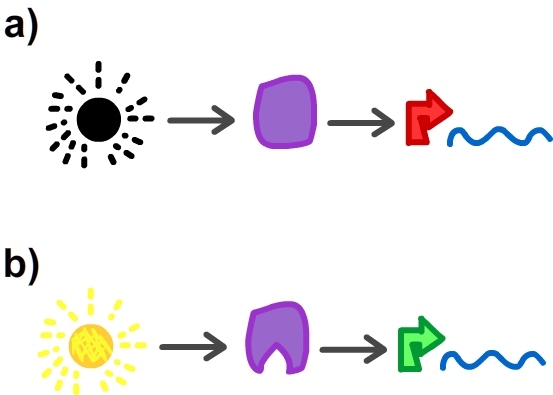
- As explained in the Overview section, we desire the input to the cell to be light. Because light cannot alone activate or repress a promoter, the circuit must in some way be able to translate the light signal to a molecule that can regulate transcription. Additionally, using light as input implies a change in light state - from OFF to ON or vise versa. Thus, the molecule that provides the interface between light and the circuit must be able to switch between active and inactive states.
- Processing
- Given a molecule that toggles between active and inactive, it is easy to imagine a circuit that switches between one state of production and a second. However, we desire to achieve a progression of four states! In order to accomplish this, our circuit, unlike the light responsive molecule, cannot return to its original state when the light turns back OFF. Clearly, our circuit must be able to hold "memory!"
- Output
- We want our circuit to have distinct states - when possible, we would like a state to turn off after a new state has been turned on (with the exception of the memory, of course!).
Second is the Memory Module, which "remembers" that light has been changed in the past. This Memory Module is a crucial way-point in the circuit, as without it the circuit would merely return to its original state when the light turns back OFF.
Third is the IF&NOT Module that generates the third state. As this module can only be active following the first and second states, its activation must depend on the presence of memory ON and the absence of some factor produced only when the light is some α state.
Finally, the last piece of the circuit is the AND Module, which produces the fourth and final state in our circuit. This module uses AND logic because it requires two inputs: one that is only expressed when the third state is active and a second that is expressed when the light is in some β state (the opposite of the previous light state, for the user has inputted a change!).
Contents |
Conversion Module
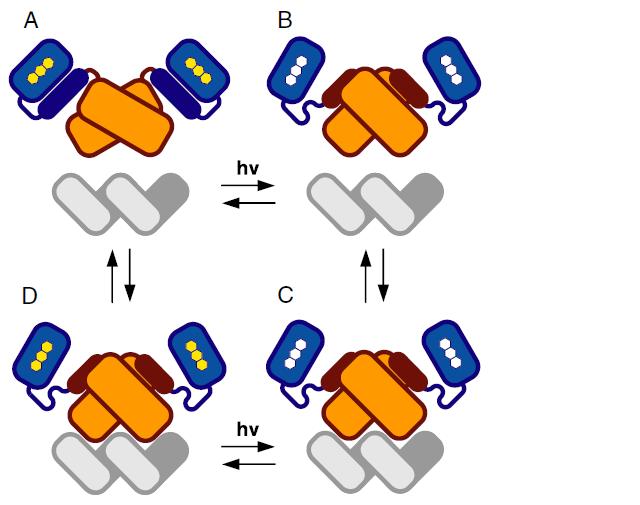
In order to design the Conversion Module, which converts light ON/OFF to transcription ON/OFF, we looked to past years' iGEM team projects. We found (quite easily, in fact, as it won best new part) team EPF-Lausanne 2009's E.Colight project, which characterized a synthetic protein LovTAP capable of acting as a repressor like tryptophan when exposed to 470nm light. You can read more about their project here. As EPF-Lausanne describes, "Upon light induction, a conformational change is transmitted from the light-sensitive to the DNA-binding domain, which ultimately results in an increase of the regulatory domain's affinity for its respective DNA binding site." This sounds like a match for what we desire! This LovTAP molecule is changed shape under exposure to light, and changes back to its original form when that light is removed. Furthermore, this part has been well characterized. Part of this characterization by the EPF-Lausanne team involved the creation of a read out system, which undergoes a change in transcriptional output in response to the changing conformation of the LovTAP protein under 470nm light. Thus, contained in EPF-Lausanne's work from last year is the essentials of the Conversion Module: <partinfo>BBa_K191003</partinfo> generates the LovTAP protein and <partinfo>BBa_K191005</partinfo> is the response system to transmit the conformational change of LovTAP to a change in transcriptional output. <partinfo>BBa_K191003 SequenceAndFeatures</partinfo> <partinfo>BBa_K191005 SequenceAndFeatures</partinfo>This system functions as follows: LovTAP is produced (given IPTG is in the culture - this must be changed for the final product to a constitutive promoter) and is expressed to saturation in the cell. When the cell is not exposed to 470nm(light), the TrpR promotor is on, and TetR is expressed, which represses the p(TetR) promotor. Thus, there is no expression the proteins downstream of p(TetR) when the light is OFF. When the light is ON, LovTAP changes conformation to its active form and represses the TrpR promoter. This ceases expression of TetR, which in turn no longer represses p(TetR), and the protein downstream of p(TetR) is expressed. This general system forms the sort of "grand central" of our circuit, and we will revisit it later with the modifications made to it by our team when considering the circuit in whole.
Memory Module
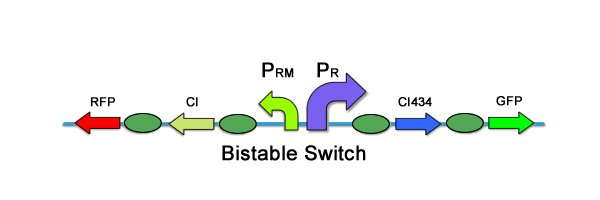
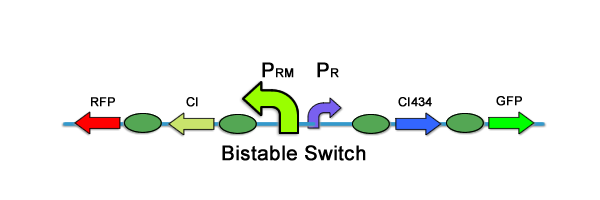
The memory module has the requirement of switching from one, original state, to a new state given some input. Furthermore, it has to remain in that new state, even if the input is taken away, thus "remembering" what has occurred. Again, we looked to past teams' work, and found the bistable switch of team PKU '07 to be a perfect match for what was required. The following two illustrations are taken from PKU 2009: The bistable switch has two components that give rise to the two distinct states. In the "no memory" state, or original state, the switch predominantly produces CI434, a repressor of the PRM promoter. Thus, the system is dominated by the PR promoter, and GFP is produced, signifying state one (and no memory). However, when CI is introduced, the PR promoter is repressed and the PRM activated. Because expression of CI is also controlled by the PRM promoter, this acts as a positive feedback loop to stably switch the bistable part to state two, where RFP is produced. Furthermore, regardless of whether or not CI is no longer externally added to this system, the bistable switch will remain in its "memory" state, or state two.IF&NOT Module
This module involves conditional logic designed to elicit the third state only after a light change occurs following activation of the second state. Because the second state (the memory gaining of the bistable switch) occurs when the light turns ON, the third state must occur when the light turns OFF. Thus our conditional must depend on:
- an inducer produced in the second state
- either an inducer produced when the light is OFF or a repressor produced when the light is ON
In the case where the third state is triggered in part by an inducer produced when the light is OFF, logic points to an AND gate. In the case where the third state is triggered in part by a repressor produced when the light is ON, logic points to a hybrid IF and NOT design, where a promoter must be activated by one factor only in the absence of another.
For the third state, we opted to go with the hybrid (IF and NOT) approach, as it simplifies the number of constructs in the system (plus, team Missouri-Davidson '08 has some cool looking hybrid promoters we wanted to try out). Specifically, we used part <partinfo>BBa_K091104</partinfo> in the design of our module to achieve the proper IF&NOT logic. This pLac/Mnt hybrid is described as activated by a combination of LacI/IPTG only in the absence of Mnt; thus, LacI serves as the inducer produced in the second state (the memory ON section of the bistable switch) and Mnt the repressor expressed when the light is ON - namely, light ON component of the Conversion Module.
AND Module
This module regulates the expression/activation of the fourth and final state of the circuit. As with the third state, it is desired that the fourth state will only be triggered given prior activation of the previous states. Thus, an AND gate, where transcription is dependent upon the positive presence two transcription factors, is necessary to prevent the fourth state from occurring at the improper time. We looked at past team's attempts at AND gates, and found PKU 2009's description of the supD/T7ptag AND gate appropriate.Putting it all together
The diagram below is an interactive depiction of our circuit in its entirety. Press the LIGHT button to toggle the light ON and OFF. Note that when components are greyed out, they are "off" or repressed, and when they are black they are "on" or active. This depiction of the circuit was created using Adobe Flash CS4.
 "
"