Team:Brown/Project/Ecargo
From 2010.igem.org
E.Cargo overview
IN SUMMARY
- We tried to create a modular Tat protein transduction domain Biobrick.
- We fused this to transcription factors in RFC25 format. This would allow us to transiently deliver proteins to a cell and thus test components of our LRC.
Background
Trans-Activator of Transcription (Tat) is an HIV gene that functions to increase the transcription level of viral genes. The Tat Protein Transduction domain (Tat-PTD) is an 11-amino acid peptide (YGRKKRRQRRR) that allows the protein to traverse cell and nuclear membranes in order to localize to the nucleus.
As a result of years of study, it is now known that there are multiple methods by which this domain operates. Most commonly, the Tat-PTD interacts with heparin sulfate proteogycans (HSPGs) to cause lipid-raft mediated endocytosis. In the endocytosed vesicles, heparinase causes degradation of the heparin sulfate, which releases the bound Tat-PTD (and anything attached to it) into the cytoplasm1. Tat-PTD has also shown the capability to cause nuclear localization of fused proteins3, underscoring its utility in transporting proteins, such as DNA-binding transcription factors, which must localize to chromatin in order to have any effect on the cell.
Rationale and Project Evolution
Our Tat-PTD project was originally developed as a way of delivering various fluorescent single-chain variable fragments (scFvs) extracted from an antibody library to mammalian cells in order to provide a staining mechanism that would allow visualization of cell structures in vitro without requiring fixation of the cells.
In the first month of summer, we reached a point where we were ready to express Tat-PTD fusion proteins, but were stalled waiting for a response from our university's Environmental Health and Safety committee as to BSL2 precautions and lab space. During that time, we focused our efforts into the numerous other projects that we were pursuing at that time. The Light-pattern-control project was designed in that period, and as it developed into a flagstone project, we struggled to manage so many projects at once. We soon came up with the idea to integrate E.Cargo into the Light-Pattern project as a sub-project.
Our idea was to use Tat-PTD fused to various bacterial transcription factors involved in the Light-Pattern circuitry in order to transiently introduce transcription factors to cells that only contained some of the circuit's components. This way, we could test constructs of the circuit individually without creating a massive amount of similar bricks and reporters for testing, and without having to worry about transforming cells with many plasmids at a time. We decided to test whether we could fuse Tat to transcription factors, and use these "Tat-TFs" to "remotely" induce gene expression.
After construction of Tat-fused parts and reporter constructs had been underway for quite some time, we discovered in our literature research that E.cargo may not be able to traverse the bacterial cell wall of E.coli. We will continue with our construction and testing of the Tat-linker and reporters while searching for an alternate bacterial chassis that is compatible with our constructs.
In the meantime, we offer the modular Tat-PTD_Linker construct in protein fusion assembly (RFC25) to the iGEM community as a means of introducing proteins to eukaryotic cells. A researcher needs simply to perform a BioBrick ligation of a his-tagged protein with our E.Cargo system, and transform the resulting product into E.coli. From there, it is a simple matter of Ni-NTA purification to isolate the Tat fusion protein for direct application to cell cultures. By fusing transcription factors to E.Cargo, one can easily test small parts of a complex circuit without need for another transformation. By fusing fluorescently-tagged proteins with specific targets to E.Cargo, it becomes a simple matter to visualize localization of target proteins or cellular structures. We hope that E.Cargo will be a valuable tool to future iGEM teams in many ways.
Experimental Design
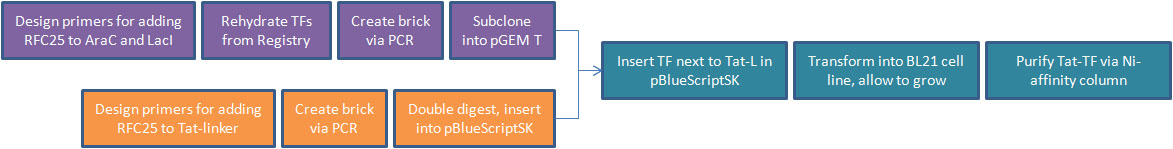
CONSTRUCTING TAT-TF
- The Tat-PTD sequence was obtained by our lab as part of a potential cell labelling project that was later dropped in the summer. We initially ordered the Tat domain with an scFv (single chain variable fragment antibody) conjugated to nuclear histone proteins. Our intention was to express this Tat-scFv fusion, purify, label with rhodamine, and apply to mammalian cells. We would've expected fluorscence to localize to nuclear histones, confirming that Tat was carrying the antibody through a live cell membrane.
- After shifting directions in our research, we decided to pursue Tat-PTD applications involving transcription factors. For our Tat-TF, we needed to create the Tat-PTD with RFC25 ends (the Freiburg assembly standard, optimized for protein fusion). We used PCR to attach sites to the existing circular Tat DNA, avoiding the process of resynthesizing. This was biobricked as our Tat-linker.
- The next step was to adapt the transcription factor for use with Tat. We decided to use two TFs from the registry, LacI and AraC. We then made a series of modifications to our proteins through PCR.
- We removed an existing LVA tag, a 3 a.a. sequence that increases rate of proteolytic degradation, because our TFs were meant to be isolated and have a long lifespan. We affixed a 6-histidine tag to facilitate purification with Nickel-affinity columns. This was done on the C-terminus to avoid isolation of incompletely translated peptides. Finally, we converted our BioBricks to RFC25.
- The linear PCR products of the transcription factors were then subcloned into pGEM-T Easy. The PCR product of the Tat-linker was double-digested and inserted into pBlueScriptSK.
- We cut the transcription factor out of pGEM-T Easy, run out on a gel to confirm successful cut, extract bands, then insert alongside the Tat-linker in pBlueScriptSK using sites for protein fusion in RFC25.
- We inserted Tat-TF/pBlueScriptSK into competent E. coli (BL21 strain) and use ampicillin resistance to select for successful transformants. NOTE: This represents the farthest we have reached in this section of our experiment.
- We will grow an overnight liquid culture of successful transformants, harvest cells and use Ni-affinity column to isolate purified Tat-TF protein.
TESTING THE TAT-TF
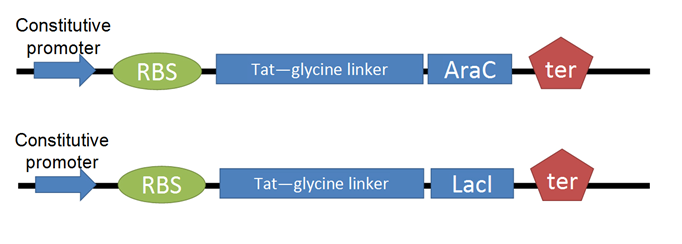
- To test for efficacy of Tat-TF control, we decided to use two reporter constructs. The LacI reporter construct was taken straight from the registry. The AraC reporter construct was assembled from registry parts:
1. Production of CyanFP is controlled by pLacI, which is constitutively on and repressible by addition of LacI protein.
2. CherryFP production is linked to pAraC, which is inducible by addition of the AraC protein.
- We then transformed each reporter construct into E.Coli (XL1B) and froze them into glycerol stocks for future use.
Results/Future Direction
- Due to the gradual evolution of this project and the time constraints of our summer, we were unable to secure experimental data on fluorescence after assembling most of our constructs in the lab. That being said, we did plan a series of experiments to determine the efficiency of our Tat-TF delivery method.
- Our first step is to confirm our reporter constructs work as expected. We created a construct that allowed cells to constitutively produce one of our transcription factors, LacI (BBa_K324003). When we transform our LacI reporter cell line with this construct, we expect LacI to be produced on site and endogenously repress cyan FP production. Quantitative analysis using a fluorometric plate reader in our research facility would measure any changes in fluorescence relative to background levels. This would be repeated with AraC to verify function of our inducible reporter.
- The next stage of our project is to test Tat-TF's ability to translocate across the cell membrane. We plan to regrow the cell lines containing our reporter constructs, apply purified Tat-TF, and measure any changes fluorescence. We expect to see a decrease in cyan fluorescence in our repressible LacI reporter, and an increase in cherry fluorescence in our inducible AraC reporter. This would indicate that our cells are responding to the remote delivery of a transcription factor.
- Finally, we will prove that the Tat domain is responsible for membrane translocation by lysing some of our cells that are constitutively producing LacI and AraC. We will apply the lysate, containing our transcription factor, to our reporter cell lines and look for changes to fluorescence. Lack of induction/repression would show that ordinary transcription factors lack the ability to freely enter the cell nucleus. This would suggest that our Tat domain functions as expected.
References
1) Chauhan A, Tikoo A, Kapur AK, Singh M. The taming of the cell penetrating domain of the HIV Tat: myths and realities. J Control Release. 2007;117:148–62.
2) Wadia JS, Dowdy SF. Protein transduction technology. Curr Opin Biotechnol. 2002;13:52–56. doi: 10.1016/S0958-1669(02)00284-7.
3) Yang, Y., Ma, J., Song, Z. and Wu, M., 2002. HIV-1 TAT-mediated protein transduction and subcellular localization using novel expression vectors. FEBS Lett. 532, pp. 36–44.
 "
"